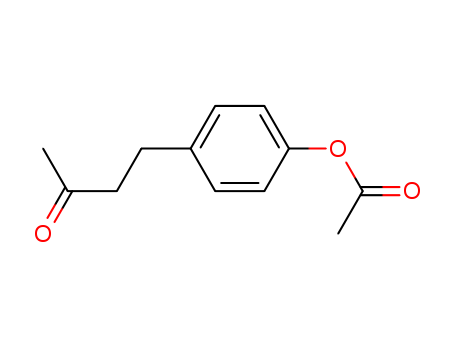
4-(4-Acetoxyphenyl)-2-butanone
-
Product Name :
4-(4-Acetoxyphenyl)-2-butanone
-
CAS No :
3572-06-3
-
Project State :
Commercial
Application
General Description
Buy cost-effective 99% pure 4-(4-Acetoxyphenyl)-2-butanone 3572-06-3 now
- Molecular Formula:C12H14O3
- Molecular Weight:206.241
- Appearance/Colour:Yellow liquid, sweet raspberry fruity odour
- Refractive Index:n20/D 1.509(lit.)
- Boiling Point:352.6 °C at 760 mmHg
- Flash Point:134.1 °C
- PSA:43.37000
- Density:1.085 g/cm3
- LogP:2.13350
4-(4-Acetoxyphenyl)-2-butanone(Cas 3572-06-3) Usage
|
Identification |
▼▲ CAS.No.:? 3572-06-3 FL.No.:? 09.288 FEMA.No.:? 3652 NAS.No.:? 3652 CoE.No.:? n/a? EINECS.No.:? 222-682-0? JECFA.No.:? 731 |
|
Regulatory Status |
CoE: n/a FDA: n/a FDA (other): n/a JECFA: ADI: Acceptable. No safety concern at current levels of intake when used as a flavoring agent (2000). |
|
Natural occurrence |
Not reported found in nature. |
|
Safety Profile |
Moderately toxic by ingestion.When heated to decomposition it emits acrid smoke andirritating fumes. |
|
Usage |
Reported uses (ppm): (FEMA, 1994) ▼▲ Food Category? Usual? Max.? Chewing.gum? 5 10 Confectionary,.frosting? 0.5 1 Fruit.ices? 0.5 1 Gelatins,.puddings? 1 2 Hard.candy? 1 2 Imitation.dairy? 0.2 1 Jams,.jellies? 0.2 1 Nonalcoholic.beverages? 0.2 0.5 Soft.candy? 0.5 2 |
|
General Description |
4-(3-Oxobutyl)phenyl acetate (4-(4-acetoxyphenyl) -2-butanone) is a standard melon fly attractant. |
InChI:InChI=1/C12H14O3/c1-9(13)3-4-11-5-7-12(8-6-11)15-10(2)14/h5-8H,3-4H2,1-2H3
3572-06-3 Relevant articles
Photocatalytic Reductive Radical-Polar Crossover for a Base-Free Corey–Seebach Reaction
Crespi, Stefano,Donabauer, Karsten,K?nig, Burkhard,Murugesan, Kathiravan,Rozman, Ur?a
supporting information, p. 12945 - 12950 (2020/09/23)
A metal-free generation of carbanion nuc...
Preparation method for synthesis of phenolic ester through thiocarboxylic acid mediated visible light catalyzed phenol acylation reaction
-
Paragraph 0079; 0080; 0100; 0101; 0102, (2018/07/30)
The invention discloses a preparation me...
Rational design, synthesis and structure-activity relationships of 4-alkoxy- and 4-acyloxy-phenylethylenethiosemicarbazone analogues as novel tyrosinase inhibitors
You, Ao,Zhou, Jie,Song, Senchuan,Zhu, Guoxun,Song, Huacan,Yi, Wei
, p. 924 - 931 (2015/03/04)
In continuing our program aimed to searc...
Synthesis of two alnustone-like natural diarylheptanoids via 4+3 strategy
Burmaoglu, Serdar,Celik, Huelya,Goeiksu, Sueleyman,Maras, Ahmet,Altundas, Ramazan,Secen, Hasan
experimental part, p. 1549 - 1562 (2009/11/30)
The first total synthesis of (4E,6E)-1,7...
3572-06-3 Process route
-
-
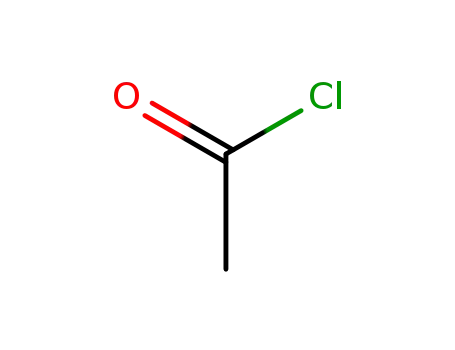
75-36-5
acetyl chloride

-
-
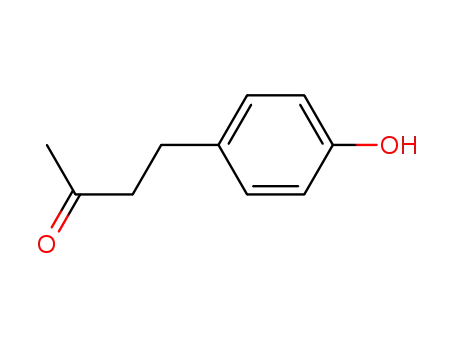
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

-
-
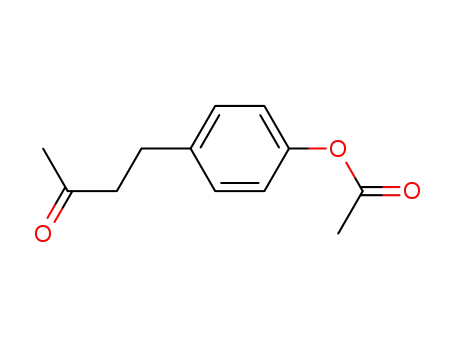
3572-06-3
4-(p-hydroxyphenyl)-2-butanone acetate
| Conditions | Yield |
|---|---|
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
|
|
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
|
-
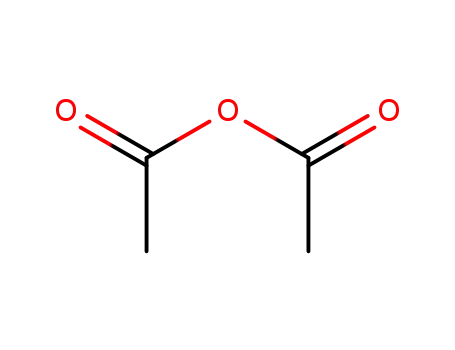
-
108-24-7
acetic anhydride

-

-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

-

-
3572-06-3
4-(p-hydroxyphenyl)-2-butanone acetate
| Conditions | Yield |
|---|---|
|
With
pyridine;
at 0 - 20 ℃;
|
80% |
3572-06-3 Upstream products
-
108-24-7

acetic anhydride
-
5471-51-2

4-(4-hydroxyphenyl)-2-oxobutane
-
75-36-5

acetyl chloride
3572-06-3 Downstream products
-
129752-69-8
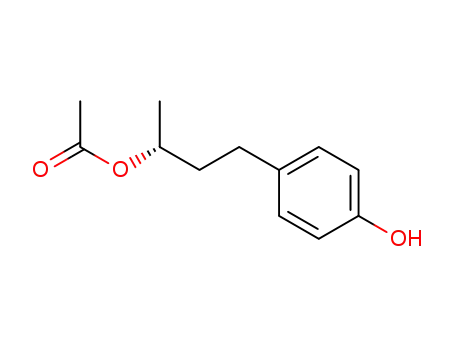
(R)-(+)-4-(4'-hydroxyphenyl)-2-butyl acetate
-
525558-18-3
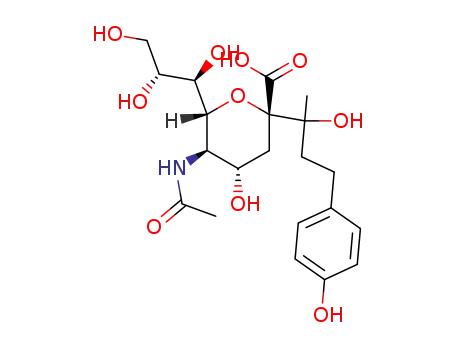
(2S,4S,5R,6R)-5-Acetylamino-4-hydroxy-2-[1-hydroxy-3-(4-hydroxy-phenyl)-1-methyl-propyl]-6-((1R,2R)-1,2,3-trihydroxy-propyl)-tetrahydro-pyran-2-carboxylic acid


