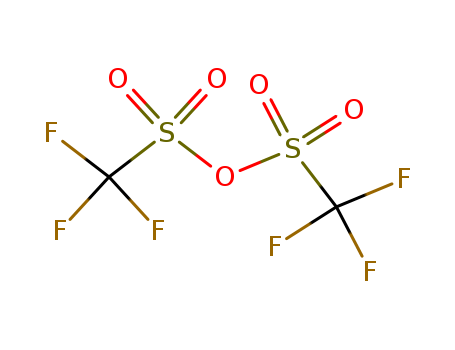
Trifluoromethanesulfonic anhydride
-
Product Name :
Trifluoromethanesulfonic anhydride
-
CAS No :
358-23-6
-
Project State :
Commercial
Application
General Description
Good factory exports good Trifluoromethanesulfonic anhydride 358-23-6
- Molecular Formula:C2F6O5S2
- Molecular Weight:282.141
- Appearance/Colour:clear colorless to light brown liquid
- Vapor Pressure:8 mm Hg ( 20 °C)
- Melting Point:-80 °C
- Refractive Index:n20/D 1.321(lit.)
- Boiling Point:82.6 °C at 760 mmHg
- Flash Point:81-83°C
- PSA:94.27000
- Density:1.976 g/cm3
- LogP:2.86380
Trifluoromethanesulfonic anhydride(Cas 358-23-6) Usage
|
Hazard |
May be corrosive to metals. Harmful if swallowed. Causes severe skin burns and eye damage. |
|
Flammability and Explosibility |
Notclassified |
|
Synthesis |
The synthesis of?Trifluoromethanesulfonic anhydride is as follows:A dry, 100-ml., round-bottomed flask is charged with 36.3 g. (0.242 mole) of trifluoromethanesulfonic acid (Note 1) and 27.3 g. (0.192 mole) of phosphorus pentoxide (Note 2). The flask is stoppered and allowed to stand at room temperature for at least 3 hours. During this period the reaction mixture changes from a slurry to a solid mass. The flask is fitted with a short-path distilling head and heated first with a stream of hot air from a heat gun and then with the flame from a small burner.The flask is heated until no more trifluoromethanesulfonic anhydride distills, b.p. 82–115°, yielding 28.4–31.2 g. (83–91%) of the anhydride, a colorless liquid. Although this product is sufficiently pure for use in the next step, the remaining acid may be removed from the anhydride by the following procedure. A slurry of 3.2 g. of phosphorus pentoxide in 31.2 g. of the crude anhydride is stirred at room temperature in a stoppered flask for 18 hours. After the reaction flask has been fitted with a short-path distilling head, it is heated with an oil bath, yielding 0.7 g. of forerun, b.p. 74–81°, followed by 27.9 g. of the pure trifluoromethanesulfonic acid anhydride, b.p. 81–84° . |
|
storage |
Store in a cool, dry, wellventilated area. ?Moisture sensitive. |
|
Purification Methods |
It can be freshly prepared from the anhydrous acid (11.5g) and P2O5 (11.5g, or half this weight) by setting aside at room temperature for 1hour, distilling off volatile products then distil it through a short Vigreux column. It is readily hydrolysed by H2O and decomposes appreciably after a few days to liberate SO2 and produce a viscous liquid. Store it dry at low temperatures. [Burdon et al. J Chem Soc 2574 1957, Beard et al. J Org Chem 38 373 1973, Beilstein 3 IV 35.] |
|
General Description |
Trifluoromethanesulfonic anhydride (triflic anhydride) is a highly reactive reagent commonly used in organic synthesis for sulfonylation, triflation, and as a strong Lewis acid catalyst. It facilitates the formation of key intermediates, such as triflates, which are essential in various transformations, including glycosylations, ring contractions, and the synthesis of complex molecules like C3-symmetric triazine derivatives and chiral oxetanes. Its electron-withdrawing properties influence reaction pathways, enabling selective bond formations and stabilizing reactive intermediates in multi-step synthetic processes. |
InChI:InChI=1/C2F6O5S2/c3-1(4,5)14(9,10)13-15(11,12)2(6,7)8
358-23-6 Relevant articles
Dilithium bis[(perfluoroalkyl)sulfonyl]diimide salts as electrolytes for rechargeable lithium batteries
Geiculescu,Xie, Yuan,Rajagopal,Creager,DesMarteau
, p. 1179 - 1185 (2004)
A series of four different dilithium sal...
Intermolecular 2 + 2 Carbonyl-Olefin Photocycloadditions Enabled by Cu(I)-Norbornene MLCT
Flores, Daniel M.,Schmidt, Valerie A.
, p. 8741 - 8745 (2019)
Photocycloadditions are often typified b...
Synthesis of 1,3-dialkyl imidazolium ionic liquids containing difunctional and tetrafunctional perfluoroalkylsulfonyl imide anions
Hickman, Tom,Desmarteau, Darryl D.
, p. 11 - 15 (2012)
Direct methylation of imidazole using me...
-
Roberts,R.M.G.
, p. 1183 - 1190 (1976)
-
Silicon Tetrakis(trifluoromethanesulfonate): A Simple Neutral Silane Acting as a Soft and Hard Lewis Superacid
Driess, Matthias,Hermannsdorfer, André
supporting information, p. 13656 - 13660 (2021/05/03)
A facile synthesis and isolation of pris...
A method of manufacturing a sulfonic acid anhydride Fluoroalkanoic (by machine translation)
-
Paragraph 0059-0061, (2017/02/23)
PROBLEM TO BE SOLVED: purpose of the pre...
METHOD FOR PRODUCING FLUOROALKANESULFONIC ANHYDRIDE
-
Paragraph 0059-0061, (2014/11/12)
Disclosed is a method for producing a fl...
358-23-6 Process route
-
-
1493-13-6
trifluorormethanesulfonic acid

-

-
75-05-8,26809-02-9
acetonitrile

-
-
1004-36-0
2,6-dimethylpyrone

-
-
7521-38-2
3-acetyl-2,6-dimethyl-4H-pyran-4-one

-
-
31111-45-2
C2H7N2(1+)

-
-
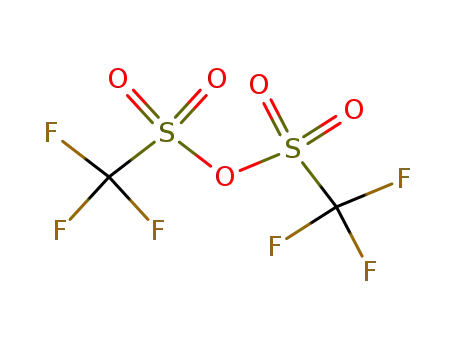
358-23-6
trifluoromethylsulfonic anhydride

-
-
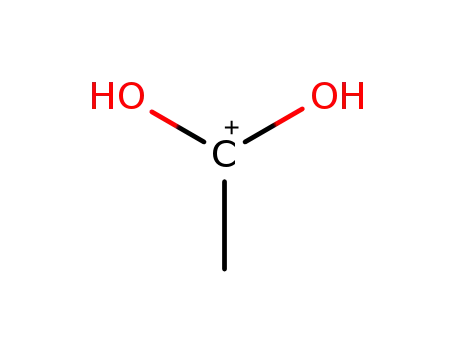
17104-45-9,18639-92-4
acetacidium

-
-
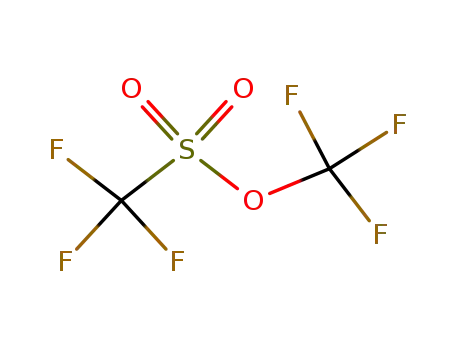
3582-05-6
trifluoromethyl trifluoromethanesulfonate

-
-
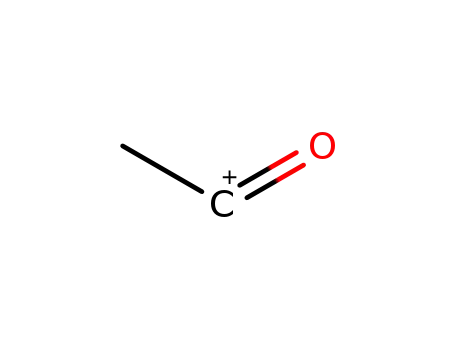
15762-07-9
oxoethylium

-
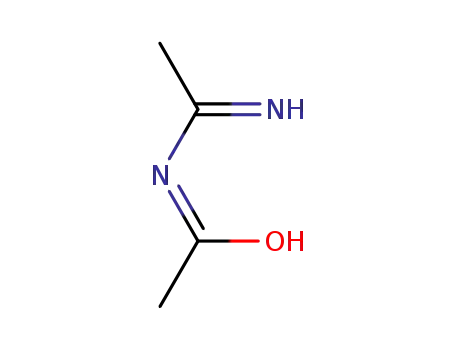
-
1361550-85-7,5699-42-3
N-acetylacetamidine

-
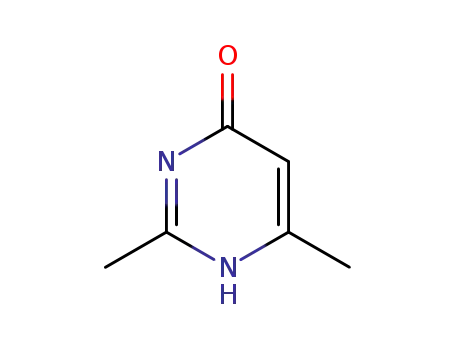
-
6622-92-0
2,4-dimethyl-1,6-dihydro-6-pyrimidone
| Conditions | Yield |
|---|---|
|
In
dichloromethane-d2;
at 74 ℃;
Cooling;
|
-
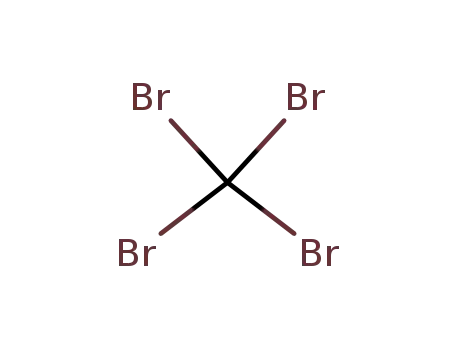
-
558-13-4
carbon tetrabromide

-
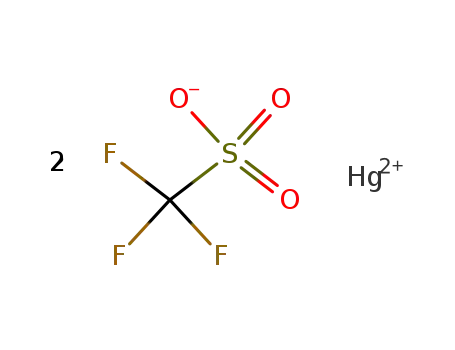
-
49539-99-3,49540-00-3
mercuric triflate

-
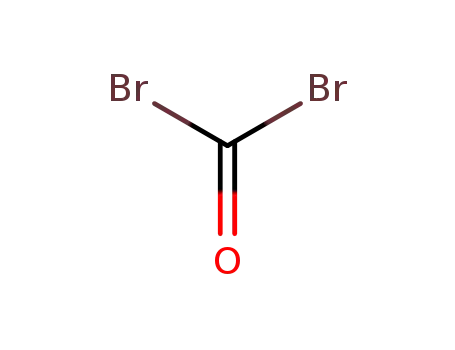
-
593-95-3
carbonyl dibromide

-

-
358-23-6
trifluoromethylsulfonic anhydride
| Conditions | Yield |
|---|---|
|
|
|
|
|
358-23-6 Upstream products
-
1493-13-6

trifluorormethanesulfonic acid
-
67-66-3

chloroform
-
65597-24-2
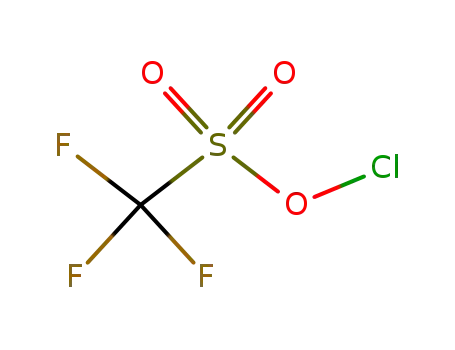
Chlorine(I) trifluoromethanesulfonate
-
75-71-8
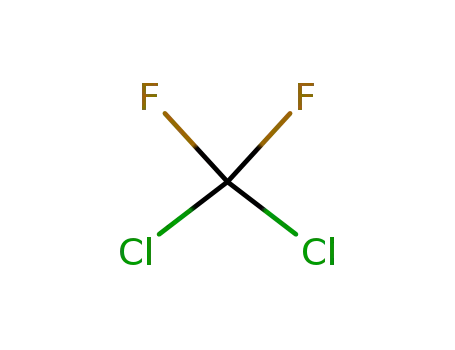
Dichlorodifluoromethane
358-23-6 Downstream products
-
28075-38-9
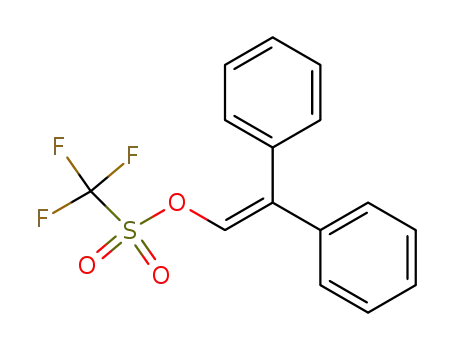
(2,2-diphenylvinyl)trifluoromethnesulfonate
-
57946-34-6
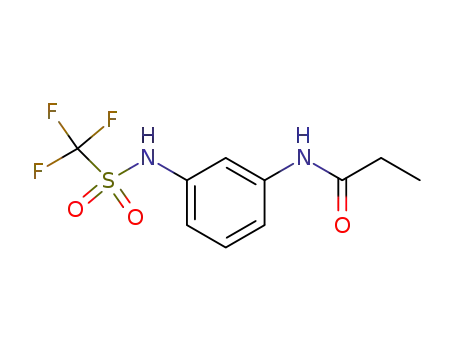
N-(3-Trifluoromethanesulfonylamino-phenyl)-propionamide
-
60306-25-4
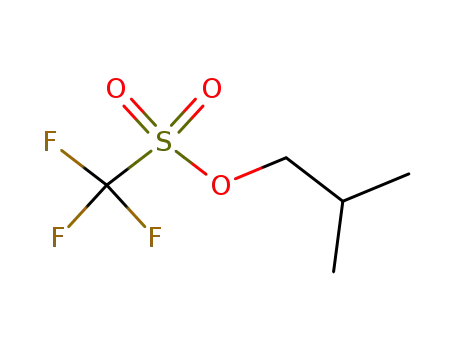
isobutyl triflate
-
64035-64-9
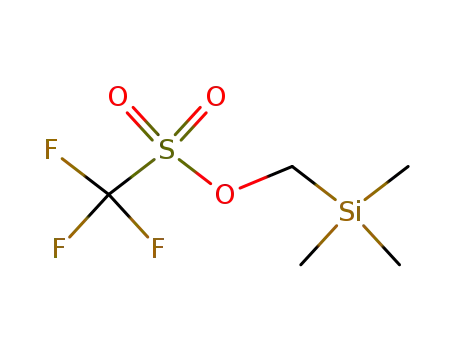
Trimethylsilylmethyl trifluoromethanesulfonate


