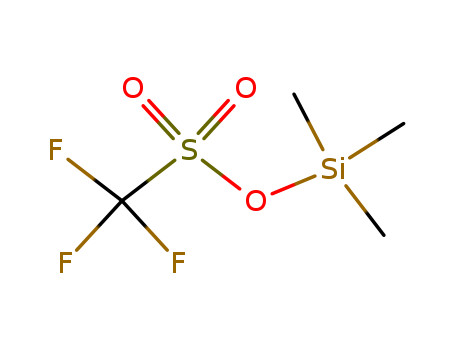
Trimethylsilyl trifluoromethanesulfonate
-
Product Name :
Trimethylsilyl trifluoromethanesulfonate
-
CAS No :
27607-77-8
-
Project State :
Commercial
Application
General Description
Cost-effective customized wholesale Trimethylsilyl trifluoromethanesulfonate 27607-77-8
- Molecular Formula:C4H9F3O3SSi
- Molecular Weight:222.26
- Appearance/Colour:clear colourless to light brown fuming liquid
- Vapor Pressure:2.22E-09mmHg at 25°C
- Melting Point:25°C
- Refractive Index:n20/D 1.36(lit.)
- Boiling Point:140 °C at 760 mmHg
- Flash Point:38.5 °C
- PSA:51.75000
- Density:1.276 g/cm3
- LogP:2.76830
Trimethylsilyl trifluoromethanesulfonate(Cas 27607-77-8) Usage
|
General Description |
Trimethylsilyl trifluoromethanesulfonate (TMSOTf) is a versatile reagent widely used in organic synthesis, acting as both a silylating agent and a Lewis acid catalyst. It facilitates reactions such as the reductive cleavage of trityl ethers, the formation of silyl ketene acetals for Mukaiyama–Mannich additions, and the stereospecific synthesis of α-glycosyl thiols. TMSOTf is particularly effective in mild, chemoselective transformations, including glycosylations and intramolecular glycosidation, where it activates substrates while tolerating acid-sensitive functional groups. Its dual reactivity makes it valuable in carbohydrate chemistry and pharmaceutical synthesis, enabling high-yielding and stereoselective reactions. |
|
Physical properties |
bp 45–47 °C/17 mmHg, 39–40 °C/12 mmHg; d 1.225 g cm?3. |
InChI:InChI=1/C18H3F35O6/c19-2(1-54,8(26,27)28)55-15(46,47)4(22,10(32,33)34)57-17(50,51)6(24,12(38,39)40)59-18(52,53)7(25,13(41,42)43)58-16(48,49)5(23,11(35,36)37)56-14(44,45)3(20,21)9(29,30)31/h54H,1H2
27607-77-8 Relevant articles
NEW METHOD FOR THE PREPARATION OF t-BUTYLDIMETHYLSILYL TRIFLATE
Hudrlik, Paul F.,Kulkarni, Ashok K.
, p. 1389 - 1390 (1985)
t-Butyldimethylsilyl triflate is easily ...
ELECTROPHILE-INITIATED SELECTIVE RING TRANSFORMATIONS OF CYCLOPROPYL KETONES
Demuth, Martin,Mikhail, Gamal
, p. 991 - 997 (1983)
Electrophile-mediated cyclopropane cleav...
REAGENTS AND SYNTHETIC METHODS 55. NEW METHODS FOR THE PREPARATION OF t-BUTYLDIMETHYLSILYL TRIFLATE AND TRIMETHYLSILYL TRIFLATE.
Aizpurua, Jesus M.,Palomo, Claudio
, p. 6113 - 6114 (1985)
An expeditious synthesis of t-butyldimet...
A Convenient in situ Preparation of Trimethylsilyl Trifluoromethanesulfonate
Demuth, Martin,Mikhail, Gamal
, p. 827 (1982)
-
Further reactions of chlorine(I) and bromine(I) trifluoromethanesulfonate and bromine(I)fluorosulfate
Johri, Kamalesh K.,Katsuhara, Yutaka,DesMarteau, Darryl D.
, p. 227 - 242 (1982)
Substitutive electrophilic dehalogenatio...
A New, Simple in Situ Preparation of Trimethylsilyl Trifluoromethanesulfonate
Ballester, Montserrat,Palomo, Antonio Luis
, p. 571 - 572 (1983)
-
Synthesis method of trimethylsilyl trifluoromethanesulfonate
-
Paragraph 0018-0023, (2021/04/29)
The invention relates to a synthesis met...
Divergent Synthesis of Vinyl-, Benzyl-, and Borylsilanes: Aryl to Alkyl 1,5-Palladium Migration/Coupling Sequences
Han, Jie-Lian,Ju, Cheng-Wei,Qin, Ying,Zhao, Dongbing
supporting information, p. 6555 - 6560 (2020/03/03)
Organosilicon compounds have been extens...
A kind of triflic acid trimethyl Estersil method for the preparation of
-
Paragraph 0113-0116, (2017/04/11)
The invention relates to a preparation m...
Redox Reactions of a Stable Dialkylphosphinyl Radical
Hirakawa, Fumiya,Ichikawa, Hitomi,Ishida, Shintaro,Iwamoto, Takeaki
supporting information, p. 2714 - 2716 (2015/06/30)
A stable dialkylphosphinyl radical, 2,2,...
27607-77-8 Process route
-
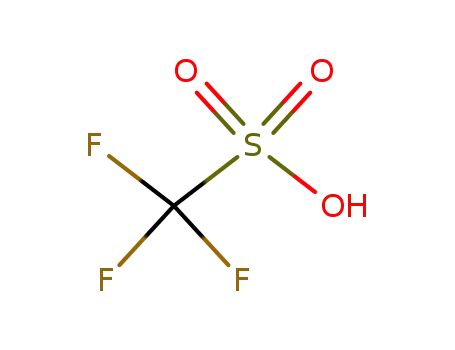
-
1493-13-6
trifluorormethanesulfonic acid

-
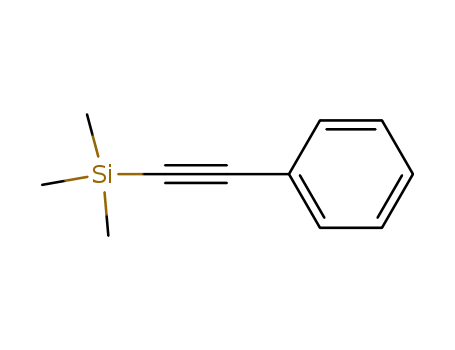
-
2170-06-1
1-Phenyl-2-(trimethylsilyl)acetylene

-
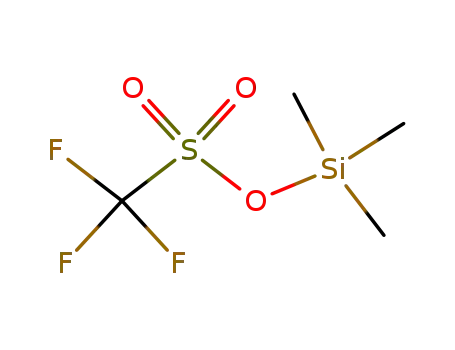
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
100-41-4,27536-89-6
ethylbenzene

-
-
1,1-Bis(triflyloxy)ethylbenzene
| Conditions | Yield |
|---|---|
|
With
HW(CO)3(C5H5);
In
dichloromethane-d2;
at 22 ℃;
for 0.833333h;
Product distribution;
other times; without Cp(CO)3WH;
|
13 % Spectr. 85 % Spectr. 91 % Spectr. |
-
-
C14H26NSi2(1+)*CF3O3S(1-)

-

-
420-56-4
trimethylsilyl fluoride

-

-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
C11H17NSi
| Conditions | Yield |
|---|---|
|
With
cesium fluoride;
In
N,N,N,N,N,N-hexamethylphosphoric triamide;
|
27607-77-8 Upstream products
-
75-77-4
chloro-trimethyl-silane
-
2923-28-6
silver trifluoromethanesulfonate
-
107-46-0
Hexamethyldisiloxane
-
358-23-6
trifluoromethylsulfonic anhydride
27607-77-8 Downstream products
-
70973-29-4
1-trimethylsiloxymethylcyclohexene
-
72166-05-3
2'-cyclohexyl-3β-methoxy-4'ξ-(trimethylsilanyloxy-methyl)-(5α,16β,17β)-tetrahydro-androstano[16,17-e][1,2]oxazine-3'ξ-carbonitrile
-
24082-11-9
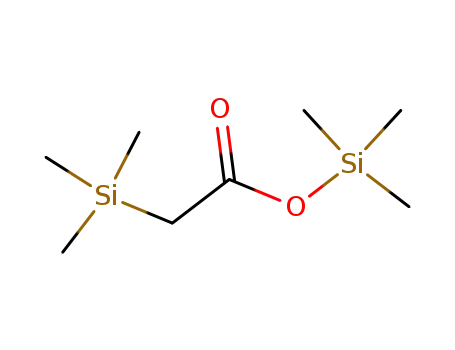
α-trimethylsilyl trimethylsilylacetic ester
-
65946-59-0
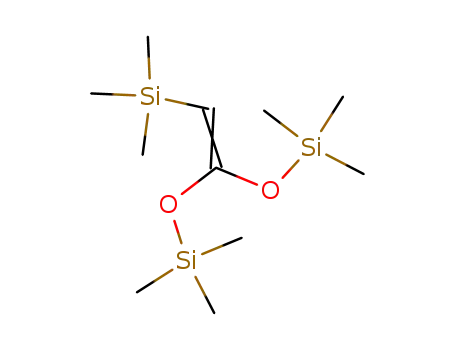
(trimethylsilyl)ketene bis(trimethylsilyl) acetal


