E3,Z8,Z11-Tetradecatriene acetate
-
Product Name :
E3,Z8,Z11-Tetradecatriene acetate
-
CAS No :
163041-94-9
-
Project State :
Commercial
Application
General Description
Bulk supply high purity E3,Z8,Z11-Tetradecatriene acetate 163041-94-9, Paid sample available
- Molecular Formula:C16H26O2
- Molecular Weight:250.3764
- Boiling Point:333.6±31.0 °C(Predicted)
- PSA:26.30000
- Density:0.903±0.06 g/cm3(Predicted)
- LogP:4.92620
E3,Z8,Z11-Tetradecatriene acetate(Cas 163041-94-9) Usage
InChI:InChI=1S/C16H26O2/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-18-16(2)17/h10-15H,3-9H2,1-2H3
163041-94-9 Relevant articles
Titanium(II)-based Z-reduction of alkynes: Stereo- and regio-specific Z-dideuteriation of conjugated and methylene-skipped ynes
Hungerford, Natasha L.,Kitching, William
, p. 1697 - 1698 (1996)
A TiII-mediated, stereo- and regio-speci...
A new and efficient synthesis of (3E,8Z,11Z)-tetradeca-3,8,11-trienyl acetate, the major sex pheromone component of the tomato leafminer Tuta absoluta
Cabezas, Jorge A.
, p. 407 - 410 (2019)
An efficient synthesis of (3E,8Z,11Z)-te...
Microscale, random reduction: Application to the characterization of (3E,8Z,11Z)-3,8,11-tetradecatrienyl acetate, a new lepidopteran sex pheromone
Attygalle, Athula B.,Jham, Gulab N.,Svatos, Ale,Frighetto, Rosa T. S.,Meinwald, Jerrold,Villela, Evaldo F.,Ferrara, Fernando A.,Uchoa-Fernandes, Manoel A.
, p. 5471 - 5474 (1995)
The major sex attractant released by Scr...
Method for synthesizing master-tomato-moth pheromone
-
Paragraph 0020; 0022; 0029-0030, (2020/01/11)
The invention discloses a synthetic meth...
Preparation of stereochemically pure E- and Z-alkenoic acids and their methyl esters from bicyclo[n.1.0]alkan-1-ols. Application in the synthesis of insect pheromones
Zubrytski,Kananovich,Matiushenkov
, p. 813 - 823 (2017/08/02)
Oxidative cleavage of exo- and endo-alky...
An Improved and Convenient New Synthesis of the Pheromone Components of the Tomato Leafminer Tuta absoluta
Puigmartí, Marc,Bosch, Ma Pilar,Guerrero, Angel
, p. 961 - 968 (2015/03/30)
A convenient new synthesis of the two ph...
163041-94-9 Process route
-
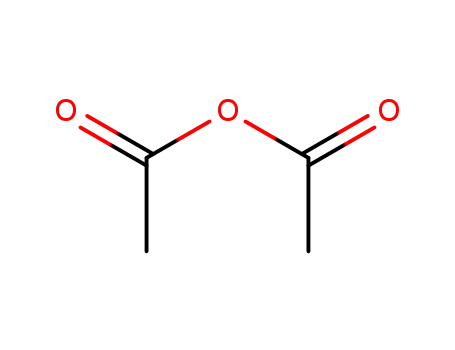
-
108-24-7
acetic anhydride

-
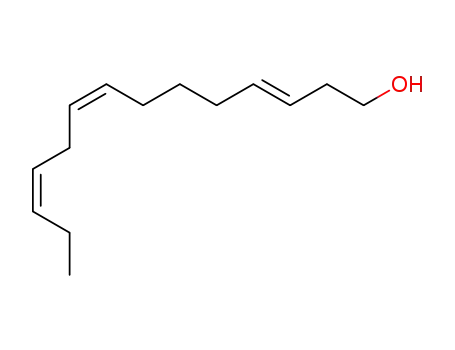
-
166901-15-1
(E3,Z8,Z11)-3,8,11-tetradecatrienyl alcohol

-
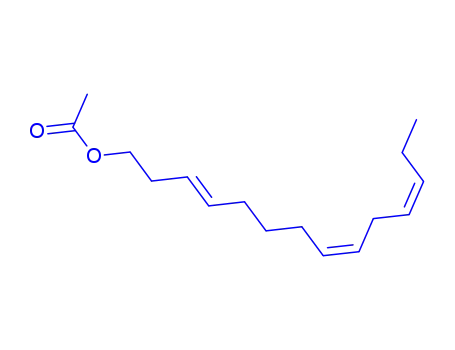
-
163041-94-9
(3E,8Z,11Z)-3,8,11-tetradecatrien-1-yl acetate
| Conditions | Yield |
|---|---|
|
With
dmap;
In
toluene;
at 50 - 70 ℃;
for 1.5h;
|
100% |
|
With
dmap; triethylamine;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 4h;
Inert atmosphere;
|
98% |
|
With
dmap; triethylamine;
In
tetrahydrofuran;
at 20 ℃;
for 4h;
stereoselective reaction;
Inert atmosphere;
|
96% |
|
With
triethylamine;
In
dichloromethane;
at 0 - 5 ℃;
for 1h;
|
80% |
|
With
pyridine;
for 1h;
|
67% |
|
In
pyridine;
Yield given;
|
|
|
With
pyridine;
|
|
|
With
pyridine;
for 1h;
Ambient temperature;
|
0.24 g |
-
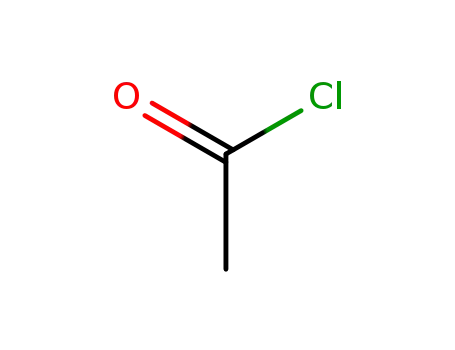
-
75-36-5
acetyl chloride

-

-
166901-15-1
(E3,Z8,Z11)-3,8,11-tetradecatrienyl alcohol

-

-
163041-94-9
(3E,8Z,11Z)-3,8,11-tetradecatrien-1-yl acetate
| Conditions | Yield |
|---|---|
|
With
triethylamine;
In
diethyl ether;
|
94% |
163041-94-9 Upstream products
-
108-24-7

acetic anhydride
-
166901-15-1

(E3,Z8,Z11)-3,8,11-tetradecatrienyl alcohol
-
62992-46-5
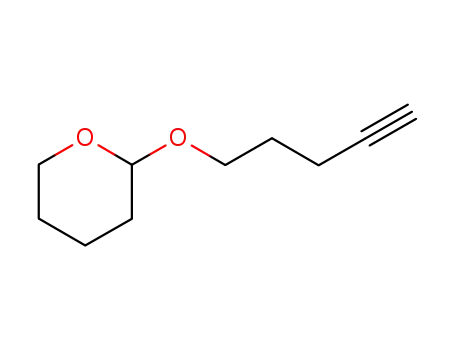
1-(tetrahydropyranyloxy)-4-pentyn
-
6261-21-8
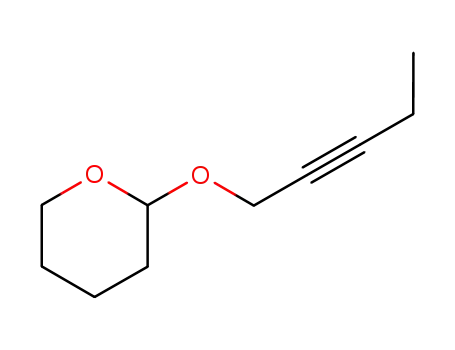
2-pent-2-ynyloxy-tetrahydro-pyran


