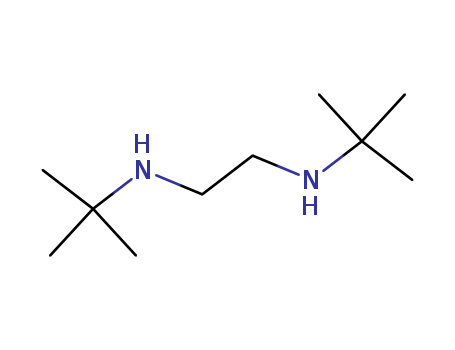
N,N'-DI-TERT-BUTYLETHYLENEDIAMINE
-
Product Name :
N,N'-DI-TERT-BUTYLETHYLENEDIAMINE
-
CAS No :
4062-60-6
-
Project State :
Commercial
Application
General Description
N,N'-DI-TERT-BUTYLETHYLENEDIAMINE 4062-60-6 with purity >99% Low price in stock
- Molecular Formula:C10H24N2
- Molecular Weight:172.314
- Appearance/Colour:COLORLESS TO YELLOW LIQUID
- Melting Point:53.35°C
- Refractive Index:n20/D 1.43(lit.)
- Boiling Point:208.7 °C at 760 mmHg
- PKA:10.49±0.38(Predicted)
- Flash Point:76.7 °C
- PSA:24.06000
- Density:0.815 g/cm3
- LogP:2.54440
4062-60-6 Relevant articles
Design, Isolation, and Spectroscopic Analysis of a Tetravalent Terbium Complex
Rice, Natalie T.,Popov, Ivan A.,Russo, Dominic R.,Bacsa, John,Batista, Enrique R.,Yang, Ping,Telser, Joshua,La Pierre, Henry S.
, p. 13222 - 13233 (2019)
Synthetic strategies to yield molecular ...
Synthesis and reactivity of the stable silylene N,N′-di-tert-butyl-1,3-diaza-2-sila-2-ylidene
Haaf,Schmedake,Paradise,West
, p. 1526 - 1533 (2000)
The synthesis and several reactions of t...
Method for synthesizing N, N-di-di-tert-butyl ethylenediamine
-
Paragraph 0016-0023, (2021/10/11)
The invention belongs to medicine. The i...
Near quantitative synthesis of urea macrocycles enabled by bulky N-substituent
Yang, Yingfeng,Ying, Hanze,Li, Zhixia,Wang, Jiang,Chen, Yingying,Luo, Binbin,Gray, Danielle L.,Ferguson, Andrew,Chen, Qian,Z, Y.,Cheng, Jianjun
, (2021/03/16)
Macrocycles are unique molecular structu...
Effect of ligand substituents in olefin polymerisation by half-sandwich titanium complexes containing monoanionic iminoimidazolidide ligands-MAO catalyst systems
Nomura, Kotohiro,Fukuda, Hiroya,Katao, Shohei,Fujiki, Michiya,Kim, Hyun Joon,Kim, Dong-Hyun,Zhang, Shu
scheme or table, p. 7842 - 7849 (2011/09/20)
Various half-sandwich titanium complexes...
4062-60-6 Process route
-
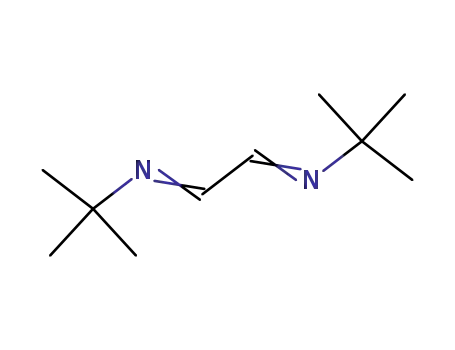
-
28227-42-1,30834-74-3
1,4-di-tert-butyl-1,4-diazabutadiene

-
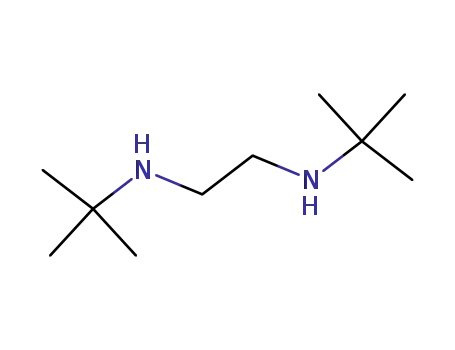
-
4062-60-6
N,N'-di-tert-butylethylenediamine
| Conditions | Yield |
|---|---|
|
With
lithium aluminium tetrahydride;
at 0 - 20 ℃;
|
89% |
|
With
sodium tetrahydroborate;
In
methanol;
at 0 - 25 ℃;
Inert atmosphere;
|
|
|
With
hydrogen; nickel;
In
toluene;
at 60 ℃;
under 22502.3 Torr;
Temperature;
Reagent/catalyst;
Autoclave;
|
-
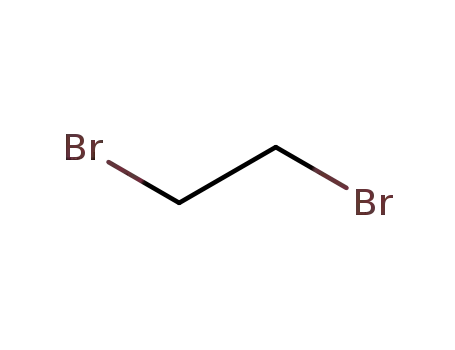
-
106-93-4
ethylene dibromide

-
-
75-64-9
tert-butylamine

-

-
4062-60-6
N,N'-di-tert-butylethylenediamine
| Conditions | Yield |
|---|---|
|
In
hexane; water;
for 72h;
Heating;
|
72% |
|
In
water;
1.) 0 deg C to ambient temperature (slowly), 2.) reflux, overnight;
|
70% |
|
ethylene dibromide; tert-butylamine;
With
sodium hydroxide;
In
water;
at 0 ℃;
for 1h;
In
water;
at 25 ℃;
for 72h;
|
56% |
|
With
water;
|
|
|
|
4062-60-6 Upstream products
-
106-93-4

ethylene dibromide
-
75-64-9

tert-butylamine
-
107-06-2
1,2-dichloro-ethane
-
30834-74-3
N-((E,2E)-2-{[(E)-1,1-dimethylethyl]imino}ethylidene)-2-methyl-2-propanamine
4062-60-6 Downstream products
-
854929-38-7
1,3-di-tert-butyl-1,3,2-diazaphospholidine-2-oxide
-
67104-96-5
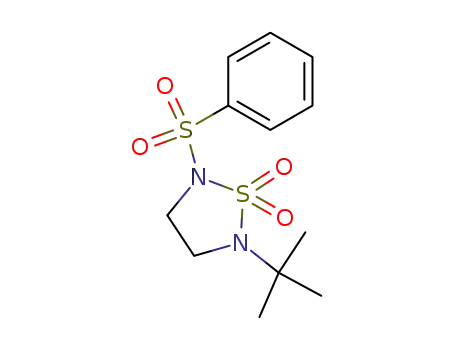
2-benzenesulfonyl-5-tert-butyl-[1,2,5]thiadiazolidine 1,1-dioxide
-
99727-73-8
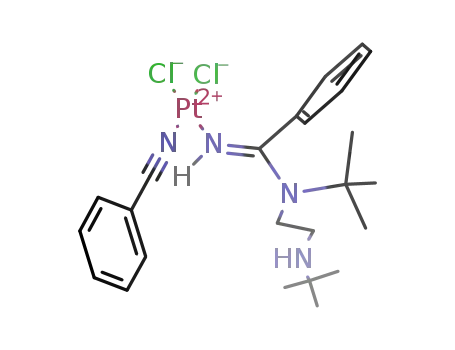
[Pt(NHC(C6H5)N(C4H9)CH2CH2NH(C4H9))Cl2(NC(C6H5))]


