octyl (R)-2-(4-chloro-2-methylphenoxy)propionate
-
Product Name :
octyl (R)-2-(4-chloro-2-methylphenoxy)propionate
-
CAS No :
66423-13-0
-
Project State :
Commercial
Application
General Description
Cost-effective and customizable octyl (R)-2-(4-chloro-2-methylphenoxy)propionate 66423-13-0 supplier
- Molecular Formula:C18H27ClO3
- Molecular Weight:326.8582
- Vapor Pressure:1.16E-06mmHg at 25°C
- Boiling Point:401.6°Cat760mmHg
- Flash Point:132.8°C
- PSA:35.53000
- Density:1.052g/cm3
- LogP:5.31940
Octyl (R)-2-(4-chloro-2-methylphenoxy)propionate(Cas 66423-13-0) Usage
|
General Description |
Octyl (R)-2-(4-chloro-2-methylphenoxy)propionate is a chemical compound commonly used as an herbicide in agricultural and commercial settings. It belongs to the class of chemicals known as aryloxyphenoxypropionate herbicides and is primarily used to control weeds and unwanted vegetation in a variety of crops, including soybeans, corn, and cotton. It works by inhibiting the growth of target plants and is known for its selective nature, meaning it primarily affects specific types of plants while leaving others unharmed. While octyl (R)-2-(4-chloro-2-methylphenoxy)propionate is effective in weed control, it is important to use it with caution and follow safety guidelines to avoid negative effects on the environment and non-target organisms. |
InChI:InChI=1/C18H27ClO3/c1-4-5-6-7-8-9-12-21-18(20)15(3)22-17-11-10-16(19)13-14(17)2/h10-11,13,15H,4-9,12H2,1-3H3/t15-/m1/s1
66423-13-0 Relevant articles
Industrial production improvement process for (R)-2-(4-chlorine-2-methyl phenoxy) octyl propionate root retarder
-
Paragraph 0023; 0031-0052; 0071, (2018/11/22)
The invention discloses an industrial pr...
Preparation method of octyl (R)-2-(4-chloro-2-methylphenoxy) propionate root retarder
-
Paragraph 0034; 0035; 0094-0129; 0146; 0147; 0148, (2018/04/01)
The invention discloses a preparation me...
66423-13-0 Process route
-
-
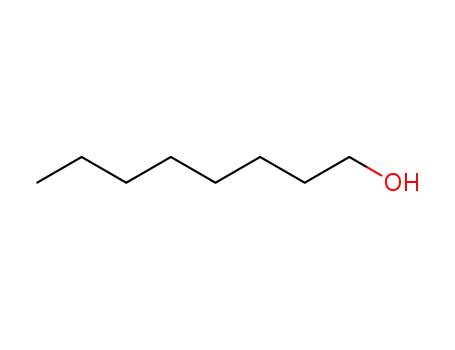
111-87-5
octanol

-
-
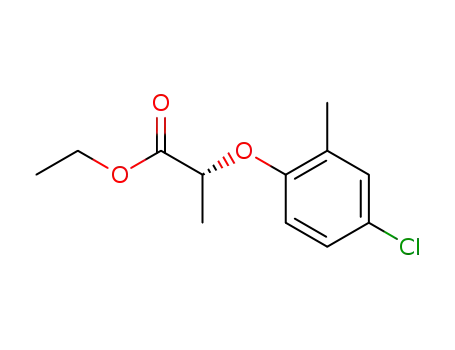
66513-22-2,40390-11-2
(R)-2-(4-chloro-2-methylphenoxy)propionic acid ethyl ester

-
-
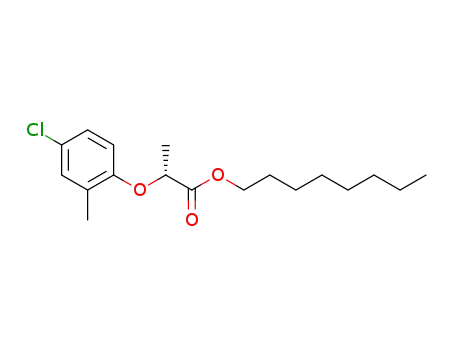
66423-13-0,161922-37-8
(R)-2-(4-chloro-2-methylphenoxy)propionic acid octyl ester
| Conditions | Yield |
|---|---|
|
With
dibutyltin dilaurate;
at 120 ℃;
Temperature;
Reagent/catalyst;
|
95% |
|
at 110 - 120 ℃;
for 0.5h;
Industrial scale;
|
85% |
-
-
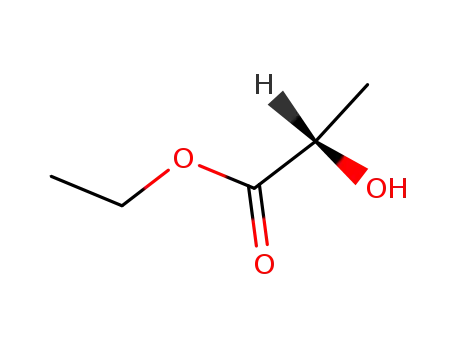
687-47-8
(S)-Ethyl lactate

-

-
66423-13-0,161922-37-8
(R)-2-(4-chloro-2-methylphenoxy)propionic acid octyl ester
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: triethylamine / toluene
2: sodium hydroxide / N,N-dimethyl-formamide
3: dibutyltin dilaurate / 120 °C
With
dibutyltin dilaurate; triethylamine; sodium hydroxide;
In
N,N-dimethyl-formamide; toluene;
|
|
|
Multi-step reaction with 3 steps
1: dmap; triethylamine / toluene / 8 h / 25 - 30 °C / Industrial scale
2: sodium hydroxide / N,N-dimethyl-formamide / Industrial scale
3: 0.5 h / 110 - 120 °C / Industrial scale
With
dmap; triethylamine; sodium hydroxide;
In
N,N-dimethyl-formamide; toluene;
|
66423-13-0 Upstream products
-
111-87-5

octanol
-
66513-22-2

(R)-2-(4-chloro-2-methylphenoxy)propionic acid ethyl ester
-
687-47-8

(S)-Ethyl lactate


