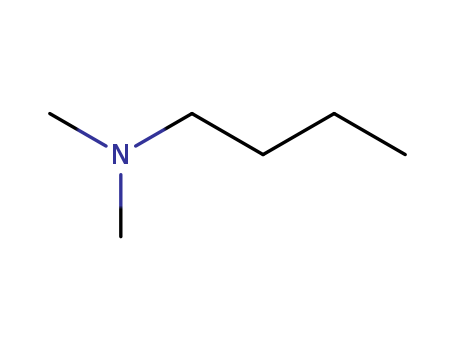
N,N-Dimethylaminobutane
-
Product Name :
N,N-Dimethylaminobutane
-
CAS No :
927-62-8
-
Project State :
Commercial
Application
General Description
Good factory supply good N,N-Dimethylaminobutane 927-62-8
- Molecular Formula:C6H15N
- Molecular Weight:101.192
- Appearance/Colour:colourless liquid
- Vapor Pressure:44.6mmHg at 25°C
- Melting Point:-60 °C
- Refractive Index:n20/D 1.398(lit.)
- Boiling Point:95.9 °C at 760 mmHg
- PKA:9.83±0.28(Predicted)
- Flash Point:25°F
- PSA:3.24000
- Density:0.75 g/cm3
- LogP:1.34810
N,N-Dimethylaminobutane(Cas 927-62-8) Usage
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 106, p. 7122, 1984 DOI: 10.1021/ja00335a042 |
|
Air & Water Reactions |
Highly flammable. Partially soluble in water. |
|
Reactivity Profile |
N,N-DIMETHYL-N-BUTYLAMINE neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides. |
|
Hazard |
Moderately toxic by ingestion. Low toxic- ity by inhalation and skin contact. A moderate eye irritant. |
|
Flammability and Explosibility |
Nonflammable |
|
Definition |
ChEBI: A tertiary amine consisting of n-butane having a dimethylamino substituent at the 1-position. |
|
General Description |
A clear liquid with an ammonia-like odor. Flash point 20°F. Boiling point 201°F. Density 0.72 g / cm3. Ingestion may irritate or burn the mouth, throat, esophagus and stomach. May cause nausea, vomiting and diarrhea. Inhalation of vapors may irritate the respiratory system and cause pulmonary edema. Contact with the skin may cause burns. Eye contact may cause corrosion to the eyes and contact with the vapor may temporarily blur vision. Vapors heavier than air and may travel considerable distance to a source of ignition and flash back. |
InChI:InChI=1/C6H15N/c1-4-5-6-7(2)3/h4-6H2,1-3H3/p+1
927-62-8 Relevant articles
Palladium-Assisted Amination of Olefins. A Mechanistic Study
Hegedus, Louis S.,Akermark, Bjorn,Zetterberg, Krister,Olsson, Lars F.
, p. 7122 - 7126 (1984)
The mechanism of the palladium-assisted ...
Intermittent synthesis method N and N -dimethyl n-butylamine
-
Paragraph 0031-0034, (2021/09/26)
The invention relates to N. The inventio...
Zirconium-hydride-catalyzed site-selective hydroboration of amides for the synthesis of amines: Mechanism, scope, and application
Han, Bo,Jiao, Haijun,Wu, Lipeng,Zhang, Jiong
, p. 2059 - 2067 (2021/09/02)
Developing mild and efficient catalytic ...
The selective reductive amination of aliphatic aldehydes and cycloaliphatic ketones with tetragonal zirconium dioxide as the heterogeneous catalyst
Bai, Peng,Li, Jiacong,Tong, Xinli,Wang, Shun,Zhang, Haigang,Zhang, Ming
, (2020/07/17)
A selective reductive amination of aliph...
Ionic liquid/H2O-mediated synthesis of mesoporous organic polymers and their application in methylation of amines
Yu, Xiaoxiao,Yang, Zhenzhen,Zhang, Hongye,Yu, Bo,Zhao, Yanfei,Liiu, Zhenghui,Ji, Guipeng,Liu, Zhimin
supporting information, p. 5962 - 5965 (2017/07/10)
Mesoporous Tr?ger's base-functionalized ...
927-62-8 Process route
-
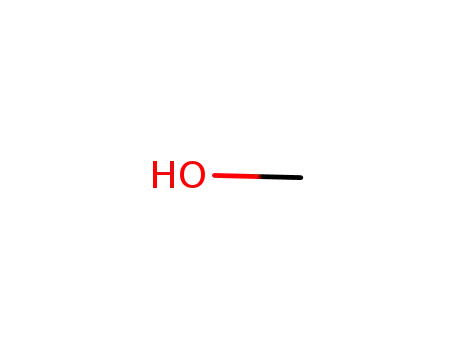
-
67-56-1
methanol

-
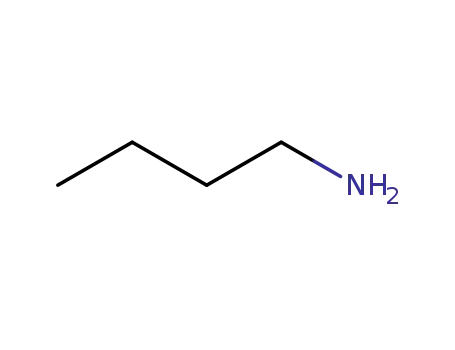
-
109-73-9,85404-21-3
N-butylamine

-
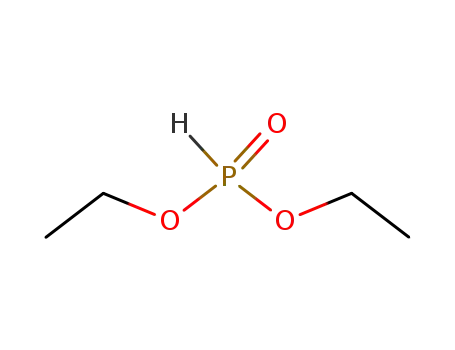
-
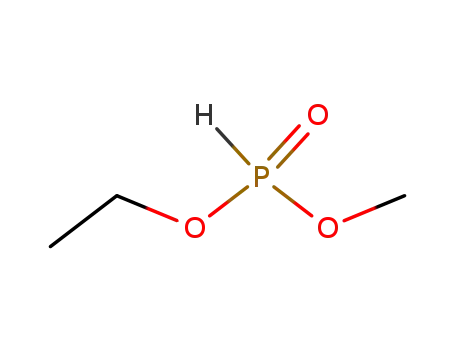
762-04-9
phosphonic acid diethyl ester

-
-
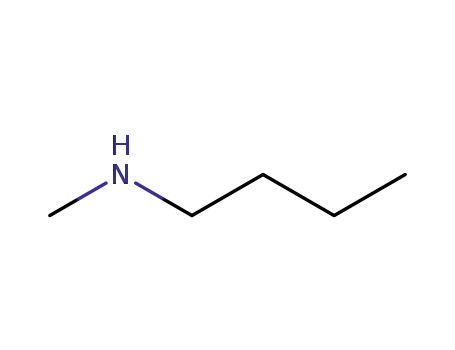
110-68-9
N-n-butyl-N-methylamine

-
-
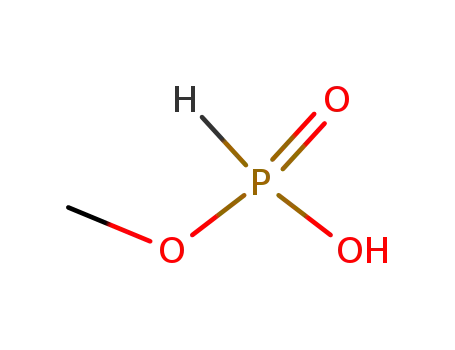
1610-33-9
O-ethyl methylphosphonic acid

-
-
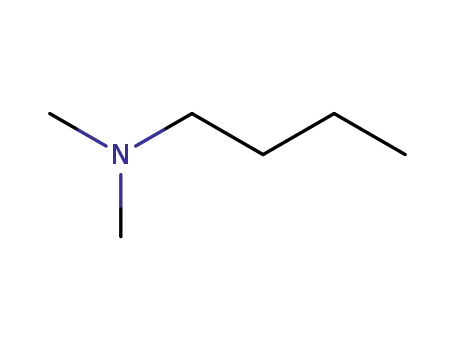
927-62-8
N,N-dimethylbutylamine

-
-
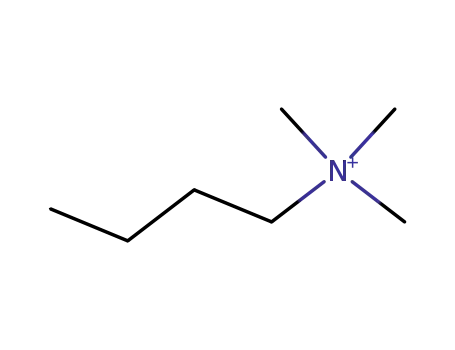
7685-30-5
N,N,N-trimethylbutylamine ion

-
-
13590-71-1
phosphonic acid monomethyl ester

-
-
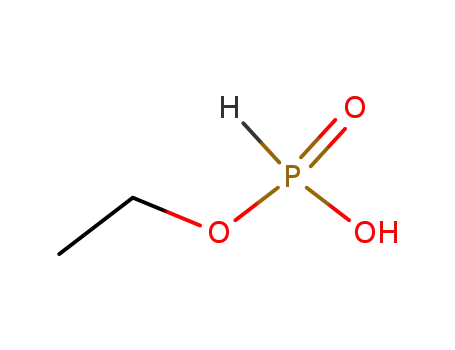
15845-66-6
Ethylphosphorige Saeure
| Conditions | Yield |
|---|---|
|
at 25 ℃;
Rate constant;
Mechanism;
other amines and dialkyl phosphites;
|
-
-
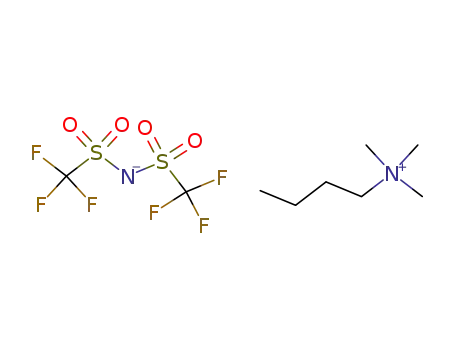
258273-75-5
butyltrimethylammonium bis(trifluoromethylsulfonyl)azanide

-
-
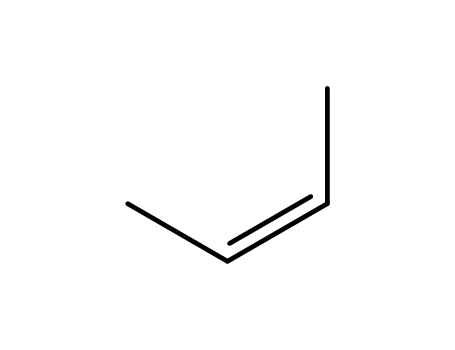
590-18-1
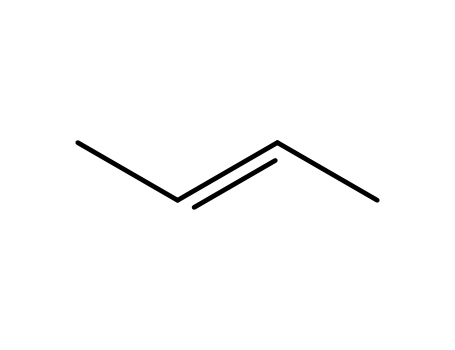
(Z)-2-Butene

-
-
34557-54-5,27936-85-2
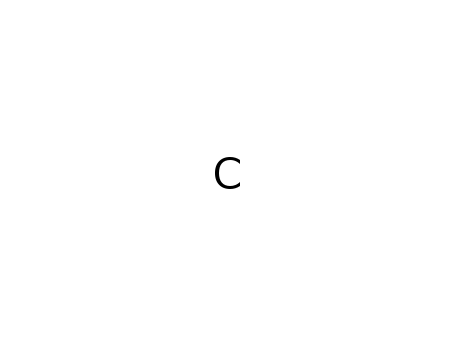
methane

-
-
624-64-6
trans-2-Butene

-

-
75-46-7
trifluoromethan

-

-
927-62-8
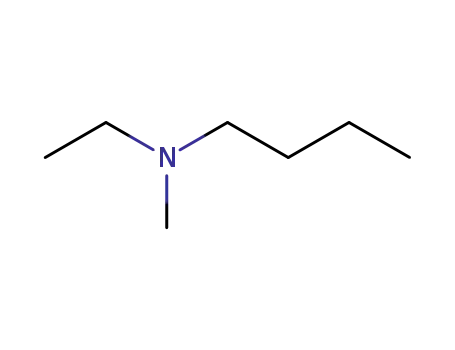
N,N-dimethylbutylamine

-
-
66225-40-9
N-ethyl-N-methylbutan-1-amine

-
-
63742-07-4
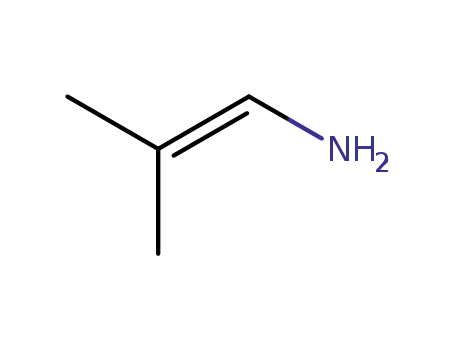
2-methylprop-1-en-1-amine

-
-
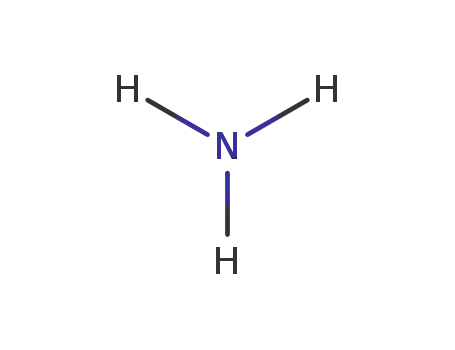
7664-41-7
ammonia

-
-
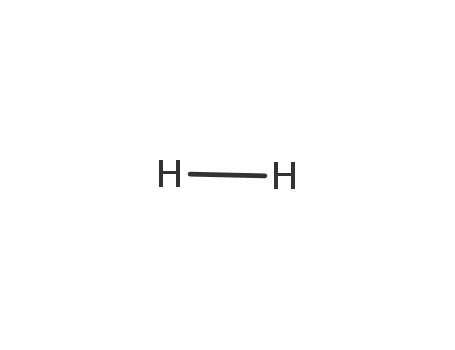
1333-74-0
hydrogen

-

-
109-73-9,85404-21-3
N-butylamine

-
-
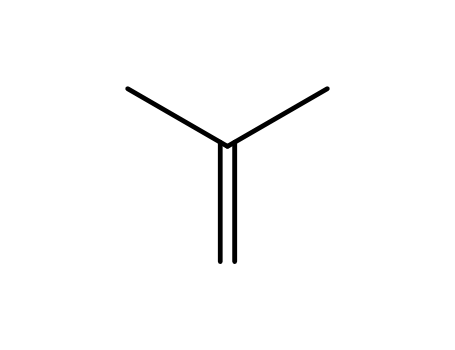
115-11-7,15220-85-6
isobutene

-

-
75-50-3
trimethylamine

-
-
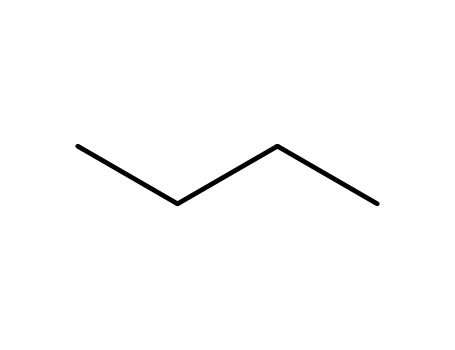
106-97-8,9003-29-6,9021-92-5
n-butane
| Conditions | Yield |
|---|---|
|
In
acetonitrile;
for 8h;
Electrochemical reaction;
|
927-62-8 Upstream products
-
50-00-0
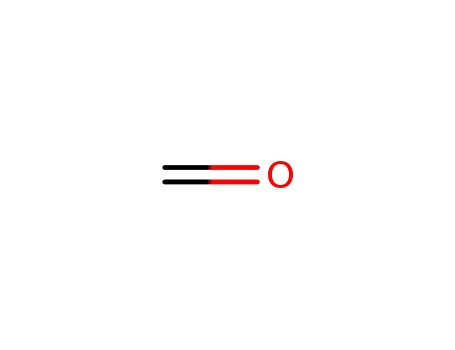
formaldehyd
-
3858-78-4
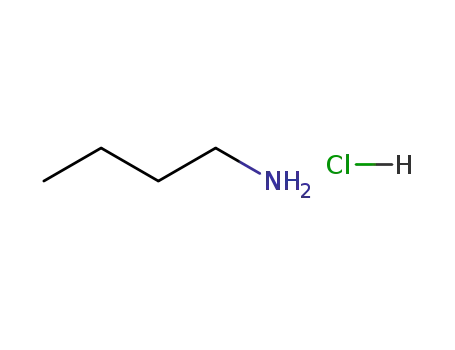
n-butylamine hydrochloride
-
7685-30-5

N,N,N-trimethylbutylamine ion
-
760-79-2
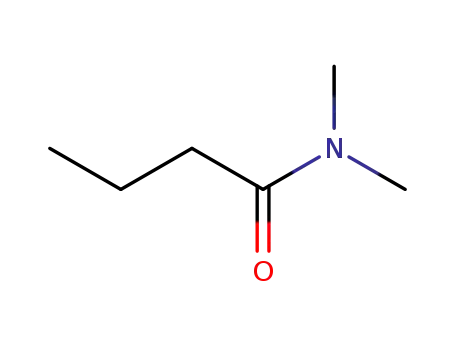
N,N-dimethylbutyramide
927-62-8 Downstream products
-
50-00-0
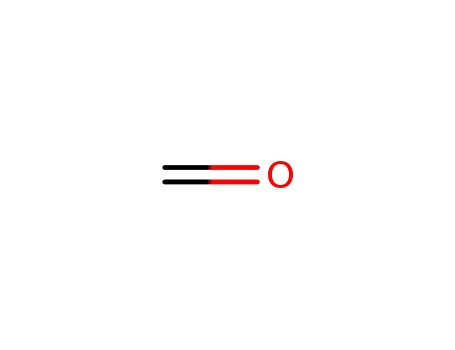
formaldehyd
-
110-68-9

N-n-butyl-N-methylamine
-
123-72-8
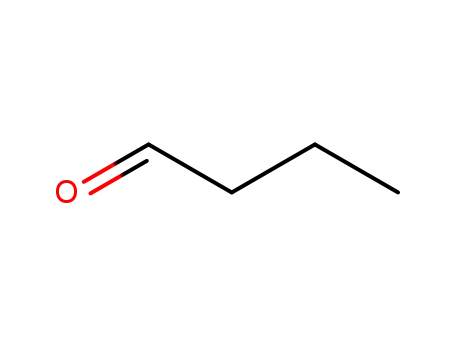
butyraldehyde
-
124-40-3
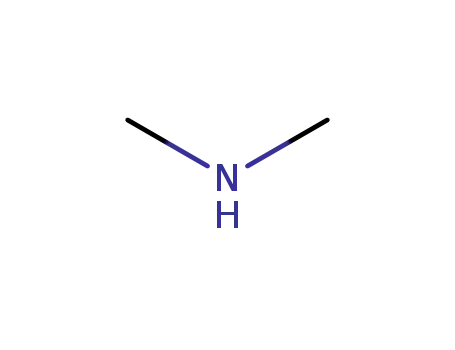
dimethyl amine


