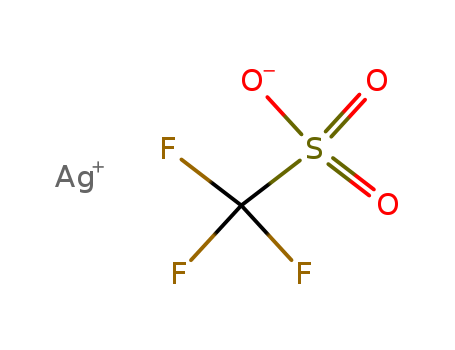
Silver trifluoromethanesulfonate
-
Product Name :
Silver trifluoromethanesulfonate
-
CAS No :
2923-28-6
-
Project State :
Commercial
Application
General Description
Cost-effective and customizable Silver trifluoromethanesulfonate 2923-28-6 factory
- Molecular Formula:CF3HSO3Ag
- Molecular Weight:256.939
- Appearance/Colour:light beige crystalline powder
- Vapor Pressure:1.14mmHg at 25°C
- Melting Point:286 °C(lit.)
- Boiling Point:162ºC at 760mmHg
- PSA:65.58000
- Density:1.876g/cm3
- LogP:1.13220
SILVER TRIFLUOROMETHANESULFONATE(Cas 2923-28-6) Usage
|
Reactions |
Silver precatalyst for the asymmetric allylation of aldehydes Silver catalyst for intramolecular additions of alcohols and carboxylic acids to inert olefins Silver catalyst for the fluorination of boronic acids Silver catalyst for the fluorination of functionalized aryl stannanes Silver catalyst for cyclopropenation of internal alkynes with donor/acceptor substituted diazo compounds Silver catalyst for the reaction of 2-alkynylbenzaldehyde with 2-isocyanoacetate |
|
Purification Methods |
Recrystallise it twice from hot CCl4 [Alo et al. J Chem Soc, Perkin Trans 1 805 1986]. Store it in the dark. [Beilstein 3 IV 34.] |
|
General Description |
Silver trifluoromethanesulfonate (AgOTf) is a versatile silver(I) salt commonly used as a cocatalyst or activator in transition metal-catalyzed reactions, particularly in gold(I)- and rhodium(I)-catalyzed processes. It facilitates the generation of cationic metal species, enhancing reactivity in cycloadditions, hydroarylations, and annulation reactions. AgOTf is also employed in glycosidation and other synthetic transformations, demonstrating its utility in forming complex organic structures, though its role may vary depending on the reaction system and conditions. |
InChI:InChI=1/CHF3O3S.Ag/c2-1(3,4)8(5,6)7;/h(H,5,6,7);/q;+1/p-1
2923-28-6 Relevant articles
Self-assembly and characterization of three-dimensional silver(I) coordination polymers containing N,N,N′,N′-tetrakis(pyridin-4-yl) methanediamine
Shin, Jong Won,Min, Kil Sik
, p. 19 - 26 (2016)
Silver(I) coordination polymers, [Ag(tpm...
1,2,4-Triazolium-5-ylidene and 1,2,4-triazol-3,5-diylidene as new ligands for transition metals
Guerret, Olivier,Solé, Stéphane,Gornitzka, Heinz,Trinquier, Georges,Bertrand, Guy
, p. 112 - 117 (2000)
Ab initio calculations show the 1,2,4-tr...
Complex formations of silver(I) trifluoroacetate and trifluoromethanesulfonate with benzene and cyclohexene
Yanagihara, Naohisa,Gotoh, Tomio,Ogura, Tetsuya
, p. 4349 - 4354 (1996)
An apparatus for measurements of equilib...
Strain-Induced Reactivity in the Dynamic Covalent Chemistry of Macrocyclic Imines
Ratjen, Lars,Vantomme, Ghislaine,Lehn, Jean-Marie
supporting information, p. 10070 - 10081 (2015/07/07)
The displacement of molecular structures...
High current density electrodeposition of silver from silver-containing liquid metal salts with pyridine-N-oxide ligands
Sniekers, Jeroen,Brooks, Neil R.,Schaltin, Stijn,Van Meervelt, Luc,Fransaer, Jan,Binnemans, Koen
, p. 1589 - 1598 (2014/01/06)
New cationic silver-containing ionic liq...
2923-28-6 Process route
-
-
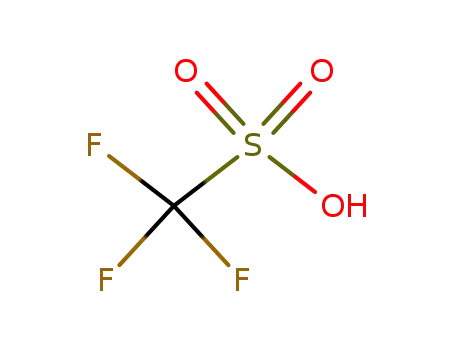
1493-13-6
trifluorormethanesulfonic acid

-

-
20667-12-3
silver(l) oxide

-
-
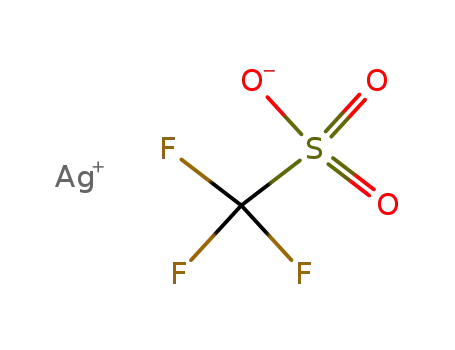
2923-28-6
silver trifluoromethanesulfonate
| Conditions | Yield |
|---|---|
|
In
tetrahydrofuran;
at 20 ℃;
for 1h;
|
95% |
|
In
methanol;
filtn., solvent removal (vac.), recrystn. (C6H6); Ag percentage detn.;
|
|
|
byproducts: H2O;
|
|
|
byproducts: H2O;
|
|
|
|
|
|
In
water;
|
-
Ag2 O
-
Ag2 O

-

-
1493-13-6
trifluorormethanesulfonic acid

-

-
2923-28-6
silver trifluoromethanesulfonate
| Conditions | Yield |
|---|---|
|
In
C6 H6; acetonitrile;
|
2923-28-6 Upstream products
-
1493-13-6

trifluorormethanesulfonic acid
-
20667-12-3

silver(l) oxide
-
563-63-3
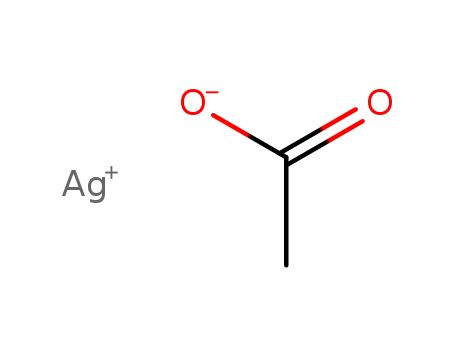
silver(I) acetate
2923-28-6 Downstream products
-
41029-44-1
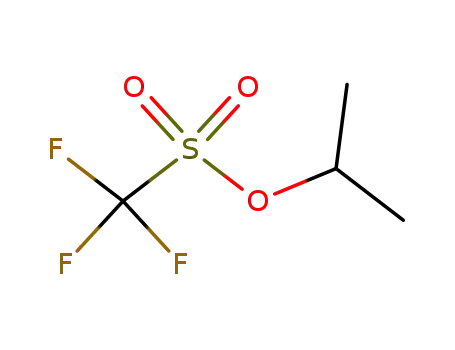
isopropyl triflate
-
27607-77-8
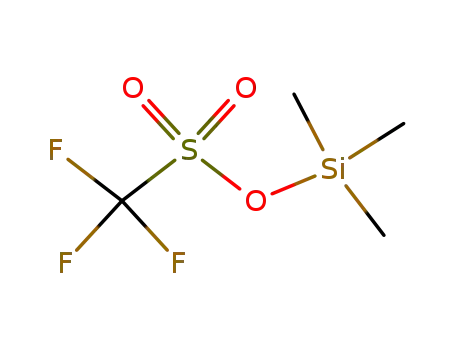
trimethylsilyl trifluoromethanesulfonate
-
27607-76-7
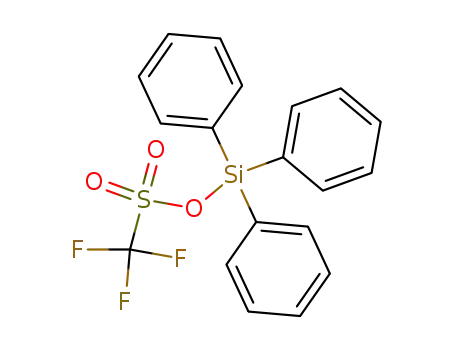
triphenylsilyl trifluoromethanesulfonate
-
55605-12-4
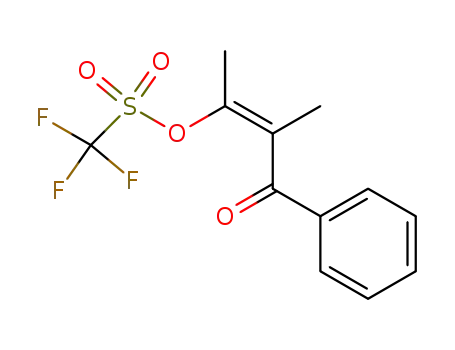
Trifluoro-methanesulfonic acid (Z)-1,2-dimethyl-3-oxo-3-phenyl-propenyl ester


