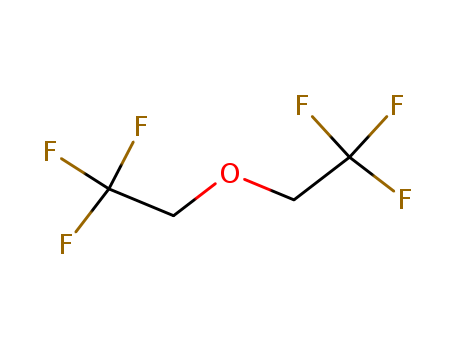
BIS(2,2,2-TRIFLUOROETHYL) ETHER
-
Product Name :
BIS(2,2,2-TRIFLUOROETHYL) ETHER
-
CAS No :
333-36-8
-
Project State :
Commercial
Application
General Description
Buy cost-effective 99% pure BIS(2,2,2-TRIFLUOROETHYL) ETHER 333-36-8 now
- Molecular Formula:C4H4 F6 O
- Molecular Weight:182.066
- Appearance/Colour:clear colorless liquid
- Vapor Pressure:180mmHg at 25°C
- Melting Point:25°C
- Refractive Index:n20/D 1.300(lit.)
- Boiling Point:63.9°Cat760mmHg
- Flash Point:1.7°C
- PSA:9.23000
- Density:1.361g/cm3
- LogP:2.12760
BIS(2,2,2-TRIFLUOROETHYL) ETHER(Cas 333-36-8) Usage
|
Manufacturing Process |
23 parts of sodium metal were placed in 300 parts of dry dioxane in a reactor equipped with an agitator and reflux condenser. The dioxane was heated to reflux while stirring.150 parts of 2,2,2-trifluoroethanol were added very slowly in the period of about 1 hour, or until the sodium was all reacted, to form sodium 2,2,2-trifluoroethylate. 250 parts of 2,2,2-trifluoroethyl ptoluenesulfonate prepared by reacting 2,2,2-trifluoroethanol with p Flurothyl toluenesulfonyl chloride were placed in another reactor and heated to about 160° to 185°C. The solution of sodium 2,2,2-trifluoroethylate in dioxane was added very slowly over a period of about 1? hours. Bis(2,2,2-trifluoroethyl) ether formed continuously and distilled from the reactor with the dioxane into a cooled receiving vessel. The condensed effluent from the reactor was fractionally distilled, yielding 46.5 parts of products boiling at 55° to 73°C.The crude product was washed successively with concentrated HCl, 62% H2SO4,concentrated H2SO4 and 5% NaOH solution. It was dehydrated over a drying agent and then refractionated in a still. 20 parts of bis(2,2,2trifluoroethyl) ether were recovered (BP 62.5° to 63.5°C). |
|
Therapeutic Function |
Central stimulant, Convulsant |
|
Brand name |
Indoklon (Ohmeda). |
InChI:InChI=1/C4H4F6O/c5-3(6,7)1-11-2-4(8,9)10/h1-2H2
333-36-8 Relevant articles
Synthesis of trifluoroethyl ethers from 2,2,2-trifluoroethyl chloride (HCFC-133a) in high temperature aqueous medium
Wu, Kai,Chen, Qing-Yun
, p. 79 - 83 (2002)
Treatment of 2,2,2-trifluoroethyl chlori...
Breaking the Trend: Insight into Unforeseen Reactivity of Alkynes in Cobalt-Catalyzed Weak Chelation-Assisted Regioselective C(4)-H Functionalization of 3-Pivaloyl Indole
Adhikari, Gopal Krushna Das,Banjare, Shyam Kumar,Dutta, Juhi,Nanda, Tanmayee,Pati, Bedadyuti Vedvyas,Ravikumar, Ponneri C.
, p. 11579 - 11587 (2021/09/22)
Unique reactivity of diphenylacetylene h...
Synthesis method and application of bis(2, 2, 2-trifluoroethyl) ether
-
Paragraph 0048-0056, (2021/02/10)
The invention provides a synthesis metho...
Synthesis of Fluorinated Dialkyl Carbonates from Carbon Dioxide as a Carbonyl Source
Sugiyama, Masafumi,Akiyama, Midori,Nishiyama, Kohei,Okazoe, Takashi,Nozaki, Kyoko
, p. 1775 - 1784 (2020/03/23)
Fluorinated dialkyl carbonates (DACs), w...
A trifluoro ethyl ether synthesis method
-
Paragraph 0017-0022, (2019/04/13)
A trifluoro ethyl ether synthesis method...
333-36-8 Process route
-
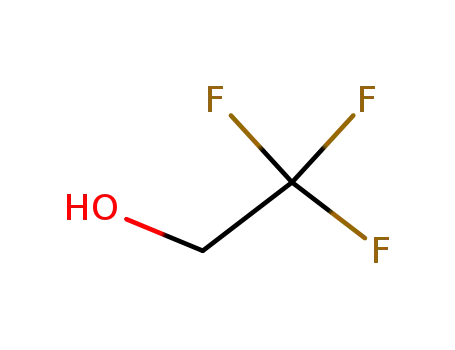
-
75-89-8
2,2,2-trifluoroethanol

-
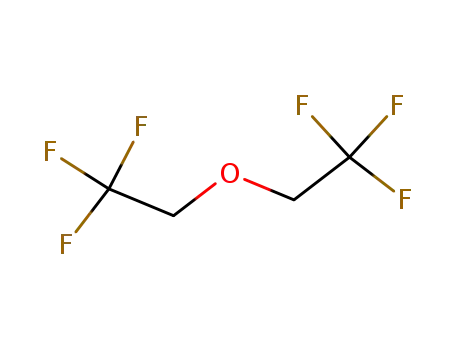
-
333-36-8
1,1,1-trifluoro-2-(2,2,2-trifluoroethoxy)ethane
| Conditions | Yield |
|---|---|
|
2,2,2-trifluoroethanol;
With
magnesium sulfate; sodium hydroxide;
at 60 ℃;
for 5h;
With
thionyl chloride;
at -5 - 50 ℃;
for 4h;
Temperature;
Inert atmosphere;
|
93.3% |
|
With
1,4-dioxane; sodium 2,2,2-trifluoroethanolate; p-toluenesulfonyl chloride;
at 160 - 185 ℃;
|
|
|
With
silver tetrafluoroborate;
at 70 ℃;
for 12h;
|
-
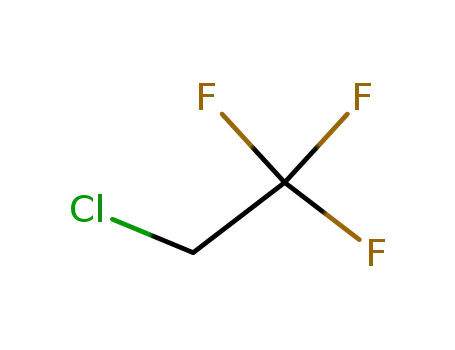
-
75-88-7
1,1,1-trifluoro-2-chloroethane

-

-
75-89-8
2,2,2-trifluoroethanol

-

-
333-36-8
1,1,1-trifluoro-2-(2,2,2-trifluoroethoxy)ethane
| Conditions | Yield |
|---|---|
|
With
potassium hydroxide;
In
1,2-dimethoxyethane;
at 70 ℃;
for 4h;
Temperature;
|
97.4% |
333-36-8 Upstream products
-
75-89-8

2,2,2-trifluoroethanol
-
110-99-6
2,2'-oxybis-acetic acid
-
421-06-7
2-bromo-1,1,1-trifluoroethane
-
298-14-6
potassium hydrogencarbonate
333-36-8 Downstream products
-
119209-26-6
1,1,1,4,4,4-Hexafluoro-2,3-butanediol bis(2,2,2-trifluoroethyl) ether
-
55605-86-2
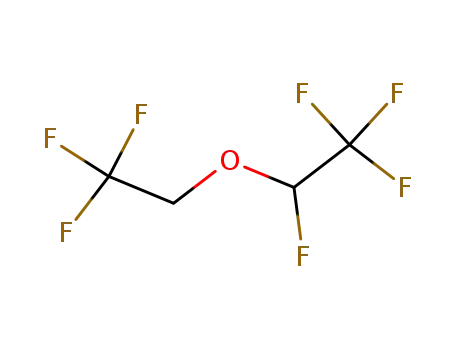
1,1,1,2-Tetrafluoro-2-(2,2,2-trifluoro-ethoxy)-ethane
-
407-38-5
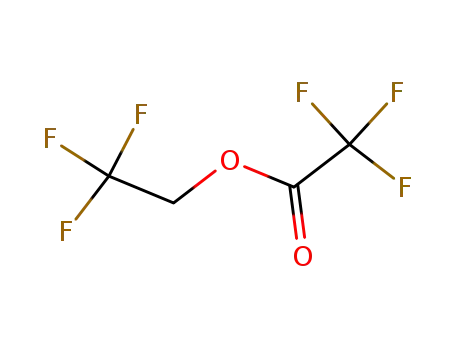
2,2,2-trifluoroethyl trifluoroacetate
-
32042-38-9
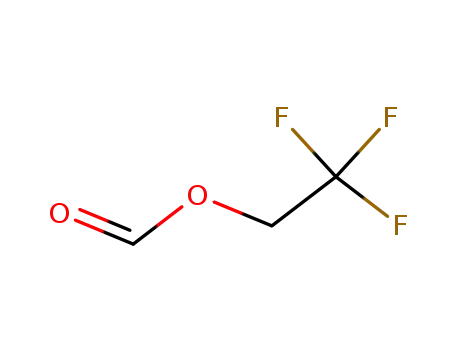
2,2,2-trifluoroethyl formate


