Vitamin E acetate
-
Product Name :
Vitamin E acetate
-
CAS No :
7695-91-2
-
Project State :
Commercial
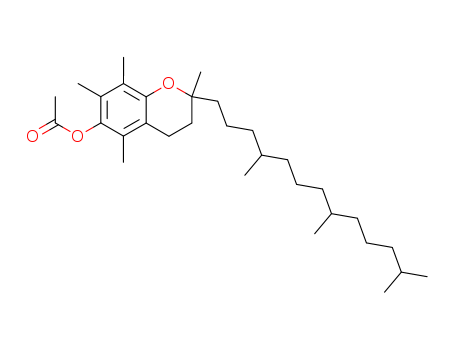
Application
General Description
Buy cost-effective 99% pure Vitamin E acetate 7695-91-2 now
- Molecular Formula:C31H52O3
- Molecular Weight:472.752
- Appearance/Colour:Clear to yellow viscous oil
- Vapor Pressure:1.42E-09mmHg at 25°C
- Melting Point:-28 °C
- Refractive Index:n20/D 1.497
- Boiling Point:485.3 °C at 760 mmHg
- Flash Point:485.3 °C at 760 mmHg
- PSA:35.53000
- Density:0.941 g/cm3
- LogP:9.05990
Tocopheryl acetate(Cas 7695-91-2) Usage
|
General Description |
DL-α-Tocopherol acetate is a stable ester form of vitamin E, widely used in the formulation of cosmetics for the prevention or correction of skin damage. |
|
Hazard |
A reproductive hazard. |
|
Flammability and Explosibility |
Nonflammable |
|
Biochem/physiol Actions |
Tocopherols are lipid soluble anti-oxidants that protect cell membranes from oxidative damage. α-Tocopherol is the form of tocopherol preferentially absorbed by Homosapiens. DL-α-Tocopherol acetate can inhibit oxidation of linoleate. |
|
Purification Methods |
It is a viscous liquid which is purified by distillation under high vacuum under N2 or Ar and stored in sealed ampoules in the dark. It is considerably more stable to light and air than the parent unacetylated vitamin. It is insoluble in H2O but freely soluble in organic solvents. All eight stereoisomers have been synthesised. The commercially pure d-tocopheryl acetate (2R,4'R,8'R) has b 180-200o/0.7mm and [] D 20 +3.9o (c 5, EtOH); see above. [Cohen et al. Helv Chim Acta 64 1158 1981, Beilstein 17/4 V 169.] |
InChI:InChI=1/C31H52O3/c1-21(2)13-10-14-22(3)15-11-16-23(4)17-12-19-31(9)20-18-28-26(7)29(33-27(8)32)24(5)25(6)30(28)34-31/h21-23H,10-20H2,1-9H3/t22-,23+,31+/m0/s1
7695-91-2 Relevant articles
SYNTHESIS OF CHROMANOL AND 2-METHYL-1,4-NAPHTHOQUINONE DERIVATIVES
-
Page/Page column 49-50; 61-62; 68-69, (2020/03/05)
The present invention relates to a proce...
Method for preparing vitamin E acetate
-
Paragraph 0068; 0069; 0070; 0071; 0073, (2019/05/15)
The invention provides a method for prep...
Vitamin E acetate preparation method (by machine translation)
-
Paragraph 0013, (2019/01/06)
The invention relates to a technical fie...
dl-alpha tocopherol acetate preparation method
-
Paragraph 0040; 0041; 0042; 0043; 0044; 0045, (2016/10/24)
The present invention relates to a dl-al...
7695-91-2 Process route
-
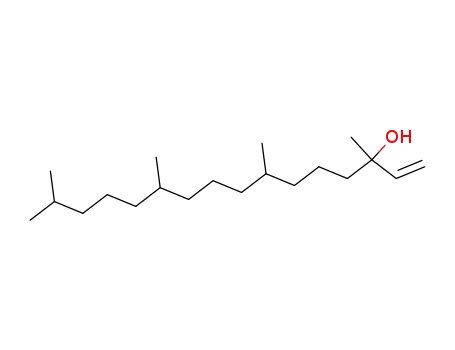
-
505-32-8
isophytol

-
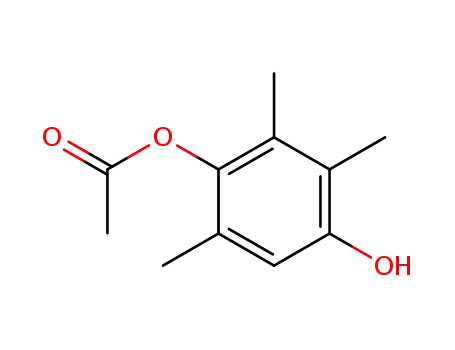
-
36592-62-8
4-acetoxy-2,3,5-trimethylphenol

-
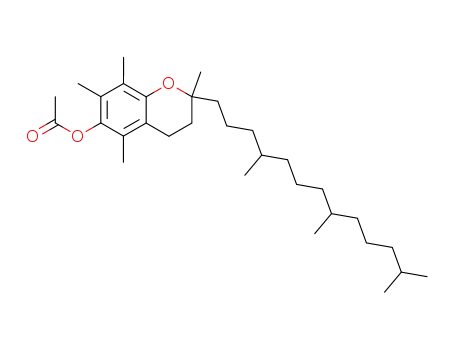
-
58-95-7,7695-91-2,18920-61-1,52225-20-4,54165-54-7,66900-46-7,79200-45-6,79200-46-7,79200-47-8,79434-84-7,79434-85-8,79434-86-9,79434-87-0,79434-88-1,104154-81-6,1406-70-8,54-22-8
vitamin E acetate
| Conditions | Yield |
|---|---|
|
trifluorormethanesulfonic acid;
In
Ethylene carbonate; n-heptane;
at 125 - 140 ℃;
for 0.5h;
Heating / reflux;
|
81.9% |
|
toluene-4-sulfonic acid;
In
Ethylene carbonate; n-heptane;
at 125 - 140 ℃;
for 0.5h;
Heating / reflux;
|
78.1% |
|
fluorosulphonic acid;
In
Ethylene carbonate; n-heptane;
at 125 - 140 ℃;
for 0.5h;
Heating / reflux;
|
67% |
|
sulfuric acid;
In
Ethylene carbonate; n-heptane;
at 125 - 140 ℃;
for 0.5h;
Heating / reflux;
|
67.5% |
|
trifluorormethanesulfonic acid;
In
toluene;
for 0.5h;
Heating / reflux;
|
62.6% |
|
trifluorormethanesulfonic acid;
In
pentan-3-one;
for 0.5h;
Heating / reflux;
|
60.7% |
|
trifluorormethanesulfonic acid;
In
acetic acid butyl ester;
for 0.5h;
Heating / reflux;
|
54.8% |
|
trifluorormethanesulfonic acid;
In
4-butanolide; acetic acid butyl ester;
for 0.5h;
Heating / reflux;
|
50% |
|
sulfuric acid;
In
4-butanolide; acetic acid butyl ester;
for 0.5h;
Heating / reflux;
|
29.5% |
|
toluene-4-sulfonic acid;
In
toluene;
for 0.5h;
Heating / reflux;
|
20.4% |
|
methanesulfonic acid;
In
Ethylene carbonate; n-heptane;
at 125 - 140 ℃;
for 0.5h;
Heating / reflux;
|
20% |
|
fluorosulphonic acid;
In
4-butanolide; acetic acid butyl ester;
for 0.5h;
Heating / reflux;
|
15.9% |
|
toluene-4-sulfonic acid;
In
4-butanolide; acetic acid butyl ester;
for 0.5h;
Heating / reflux;
|
14.2% |
|
methanesulfonic acid;
In
4-butanolide; acetic acid butyl ester;
for 0.5h;
Heating / reflux;
|
10.2% |
-

-
505-32-8
isophytol

-
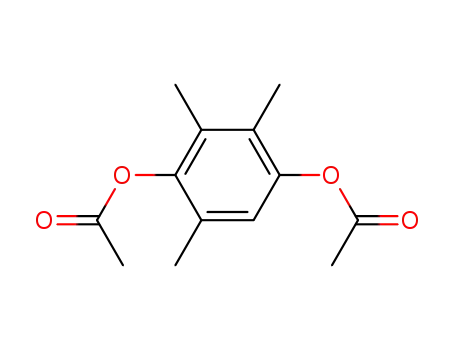
-
7479-28-9
2,3,5-trimethyl-1,4-hydroquinone diacetate

-

-
58-95-7,7695-91-2,18920-61-1,52225-20-4,54165-54-7,66900-46-7,79200-45-6,79200-46-7,79200-47-8,79434-84-7,79434-85-8,79434-86-9,79434-87-0,79434-88-1,104154-81-6,1406-70-8,54-22-8
vitamin E acetate
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; aluminum (III) chloride;
In
water; ethyl acetate;
at 60 ℃;
for 6h;
Temperature;
Reagent/catalyst;
Time;
|
94.94% |
|
With
acetic anhydride; acetic acid;
at 70 ℃;
for 3h;
Reagent/catalyst;
Temperature;
|
7695-91-2 Upstream products
-
74515-24-5

3,5,6-trimethyl-2-phytylhydroquinone
-
108-24-7
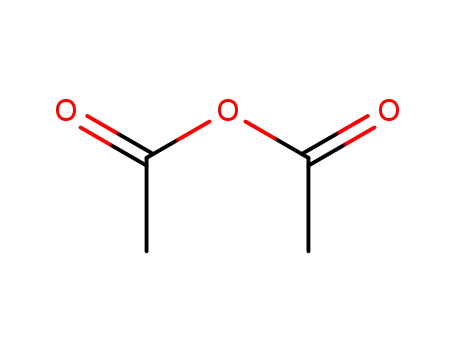
acetic anhydride
-
59-02-9
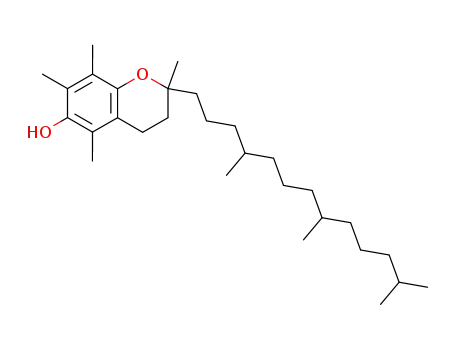
vitamin E
-
1721-51-3
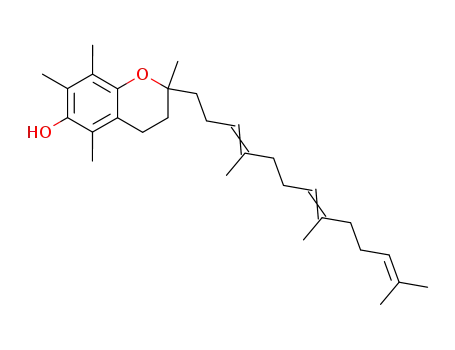
α-tocotrienol
7695-91-2 Downstream products
-
588-46-5
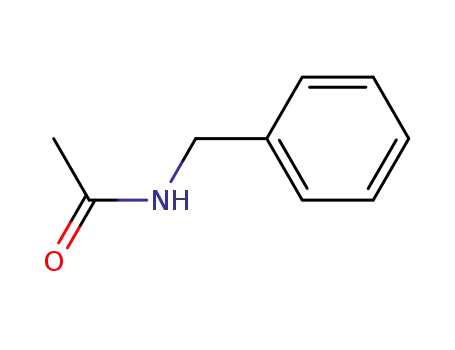
N-(phenylmethyl)acetamide
-
527-17-3
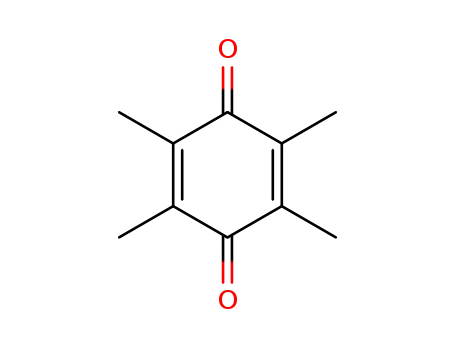
Duroquinone
-
59-02-9

vitamin E


