Synthetic Oakmoss
-
Product Name :
Synthetic Oakmoss
-
CAS No :
4707-47-5
-
Project State :
Commercial
Application
General Description
Buy high quality and low price Synthetic Oakmoss 4707-47-5 now
- Molecular Formula:C10H12O4
- Molecular Weight:196.203
- Appearance/Colour:white to pinkish yellow crystalline powder (est)
- Vapor Pressure:1.05E-05mmHg at 25°C
- Melting Point:141-146°C(lit.)
- Refractive Index:1.57
- Boiling Point:360.7 °C at 760 mmHg
- PKA:8.63±0.28(Predicted)
- Flash Point:143.9 °C
- PSA:66.76000
- Density:1.251 g/cm3
- LogP:1.50120
METHYL 2,4-DIHYDROXY-3,6-DIMETHYLBENZOATE(Cas 4707-47-5) Usage
|
Preparation |
Methyl 3,6-dimethylresorcylate is prepared by aromatization of corresponding hydroxycyclohexenones, for example, by dehydrogenation with a suitable Nhaloimide.The intermediate hydroxycyclohexenone can be obtained either by reaction of dimethylmalonate with 4-hexen-3-one and 5-chloro-3-hexanone or by reaction of methyl propionylacetate with alkyl crotonate.Another route starts from the corresponding dimethyl-1,3-dihydroxybenzene, which is carboxylated, and the resulting dihydroxymethylbenzoic acid is esterified. |
|
Flammability and Explosibility |
Notclassified |
|
Trade name |
Atralone (Agan) |
InChI:InChI=1/C10H12O4/c1-5-4-7(11)6(2)9(12)8(5)10(13)14-3/h4,11-12H,1-3H3
4707-47-5 Relevant articles
-
Fujii,Osumi
, (1936)
-
-
Koller,Poepl
, p. 126,129, 130 (1934)
-
Two new glycosides from Dianella ensifolia (L.) DC
Fan, Miao-Yin,Liu, Bing-Rui,Yang, Fan,Zhang, Pu-Zhao
, p. 18 - 20 (2021/11/11)
The Dianella genus includes approximatel...
Method for catalytically synthesizing oak moss by supported solid base
-
Paragraph 0040; 0045-0048; 0049; 0054; 0055, (2021/05/12)
The invention provides a method for cata...
Compositional variation of atranorin-related components of lichen Myelochroa leucotyliza dependent on extraction solvent and their quantitative analysis by qHNMR
Ojha, Manju,Kil, Yun-Seo,Youn, Ui Joung,Ok, Young Jun,Choi, Hyukjae,Nam, Joo-Won
, p. 1067 - 1073 (2021/04/05)
Introduction: Quantitative nuclear magne...
Method for synthesizing 2, 4-dihydroxy-3, 6-dimethyl methyl benzoate by using 4-O-demethylated barbatic acid
-
Paragraph 0089; 0096-0098, (2020/05/30)
The invention provides a method for synt...
4707-47-5 Process route
-
-
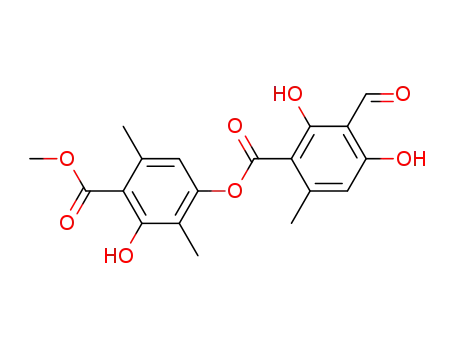
479-20-9
atranorin

-
-
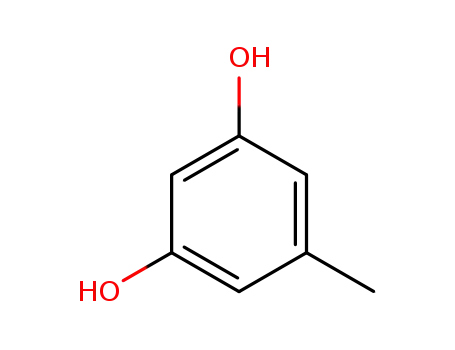
504-15-4
orcinol

-
-
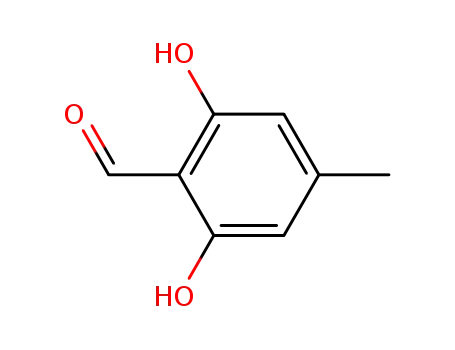
526-37-4
atranol

-
-
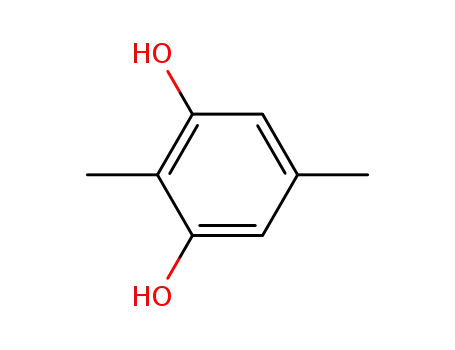
488-87-9
2,5-dimethyl-1,3-benzenediol

-
-
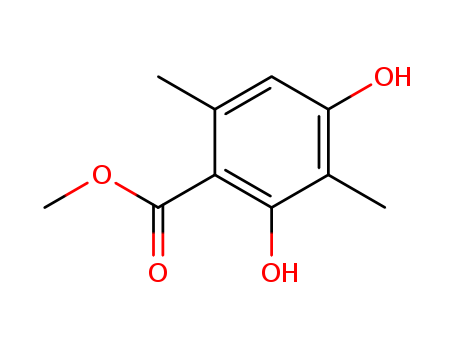
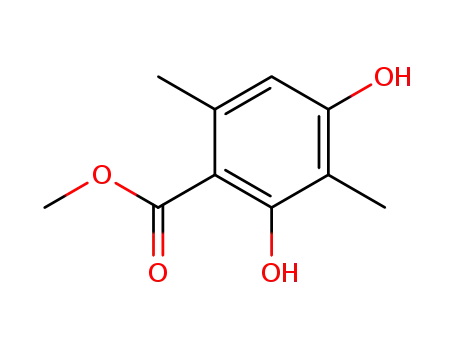
4707-47-5
2,4-dihydroxy-3,6-dimethyl-benzoic acid methyl ester

-
-
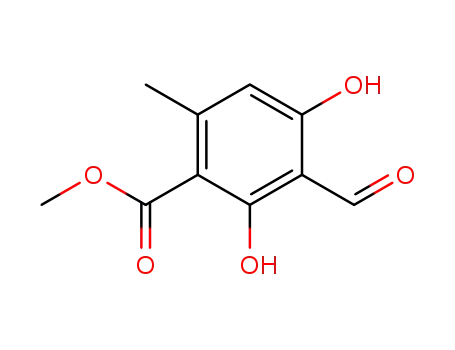
34874-90-3
methyl haematommate

-
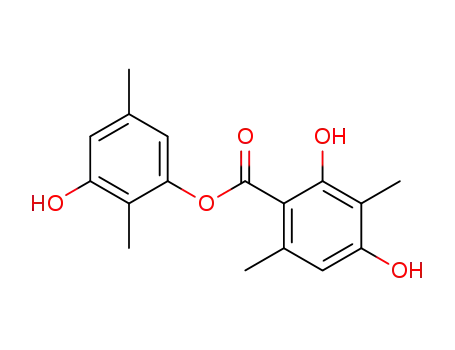
-
3-hydroxy-2,5-dimethylphenyl 2,4-dihydroxy-3,6-dimethylbenzoate
| Conditions | Yield |
|---|---|
|
at 230 - 250 ℃;
for 0.75h;
Product distribution;
pyrolysis;
|
-
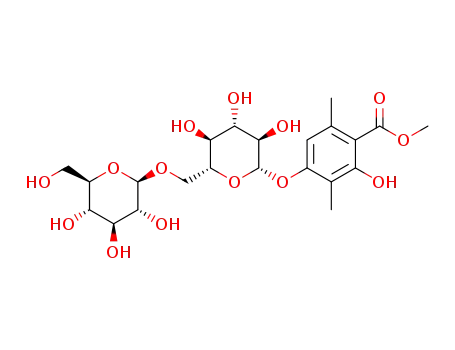
-
methyl 2-hydroxy-3,6-dimethylbenzoate-4-O-β-D-glucopyranosyl(1→6)-β-D-glucopyranoside

-
-
50-99-7
D-glucose

-

-
4707-47-5
2,4-dihydroxy-3,6-dimethyl-benzoic acid methyl ester
| Conditions | Yield |
|---|---|
|
With
sulfuric acid;
at 95 ℃;
for 5h;
|
4707-47-5 Upstream products
-
186581-53-3

diazomethane
-
4707-46-4
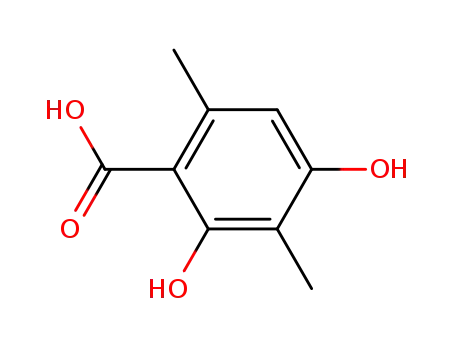
2,4-dihydroxy-3,6-dimethylbenzoic acid
-
67-56-1
methanol
-
479-16-3
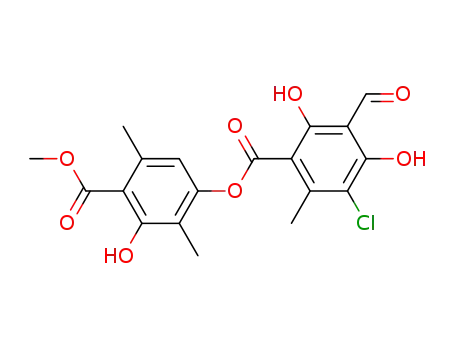
chloroatranorin
4707-47-5 Downstream products
-
81050-84-2
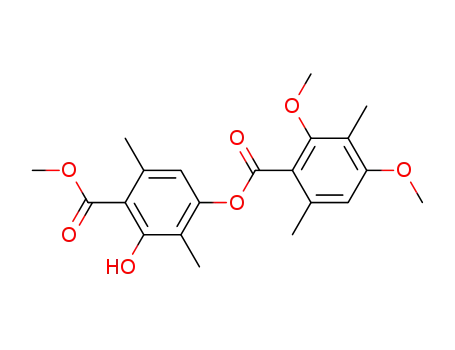
4-(2,4-dimethoxy-3,6-dimethyl-benzoyloxy)-2-hydroxy-3,6-dimethyl-benzoic acid methyl ester
-
5014-22-2
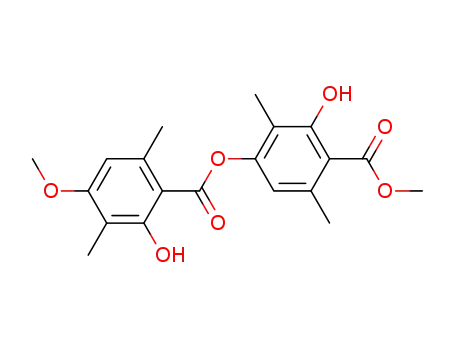
methyl barbatate
-
15222-49-8
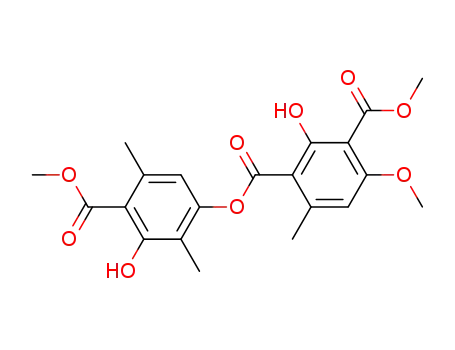
2-hydroxy-4-methoxy-6-methyl-isophthalic acid-1-(3-hydroxy-4-methoxycarbonyl-2,5-dimethyl-phenyl ester)-3-methyl ester
-
488-87-9

2,5-dimethyl-1,3-benzenediol


