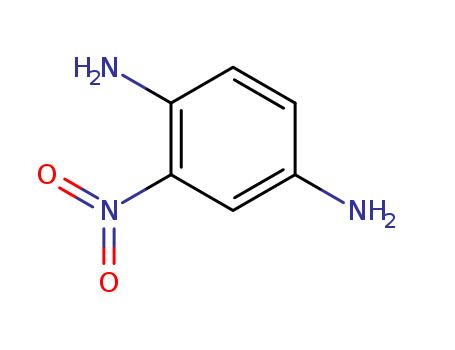
1,4-Diamino-2-nitrobenzene
-
Product Name :
1,4-Diamino-2-nitrobenzene
-
CAS No :
5307-14-2
-
Project State :
Commercial
Application
General Description
1,4-Diamino-2-nitrobenzene 5307-14-2 with purity >99% Low price in stock
- Molecular Formula:C6H7N3O2
- Molecular Weight:226.062
- Appearance/Colour:Dark red crystal powder
- Vapor Pressure:0mmHg at 25°C
- Melting Point:135-138 °C(lit.)
- Refractive Index:1.699
- Boiling Point:385.4 °C at 760 mmHg
- PKA:4.36±0.10(Predicted)
- Flash Point:186.9 °C
- PSA:97.86000
- Density:1.446 g/cm3
- LogP:2.44480
1,4-Diamino-2-nitrobenzene(Cas 5307-14-2) Usage
|
Air & Water Reactions |
1,4-Diamino-2-nitrobenzene may be sensitive to prolonged exposure to light and air. Insoluble in water. |
|
Reactivity Profile |
1,4-Diamino-2-nitrobenzene is incompatible with strong oxidizing agents. |
|
Fire Hazard |
Flash point data for 1,4-Diamino-2-nitrobenzene are not available; however, 1,4-Diamino-2-nitrobenzene is probably combustible. |
|
Flammability and Explosibility |
Nonflammable |
|
Contact allergens |
ONPD is a hair dye and a sensitizer in hairdressers and consumers who are generally sensitive to PPD too. |
|
Safety Profile |
Suspected carcinogen with experimental carcinogenic and neoplastigenic data. Poison by intraperitoneal route. Moderately toxic by ingestion. An experimental teratogen. Other experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx,. |
|
Definition |
ChEBI: A primary amino compound that is p-phenylenediamine in which one of the hydrogens attached to the benzene ring is replaced by a nitro group. It is a cosmetic hair dye intermediate that is used in permanent hair colouring products (diluted 1:1 ith an oxidising agent prior to application). |
|
General Description |
Almost black needles with dark-green luster or black powder. |
InChI:InChI=1/C6H7N3O2/c7-5-1-3-6(4-2-5)8-9(10)11/h1-4,8H,7H2
5307-14-2 Relevant articles
Protonation of Cationic Bases in Perchloric Acid: Establishment of the H+ Scale in 0-11 M Perchloric Acid
Lovell, Michael W.,Vogt, Brian S.,Schulman, Stephen G.
, p. 1885 - 1888 (1984)
The protonation of several monocationic ...
[1+1] Copper(II) macrocyclic Schiff base complex on rGO as a photocatalyst for reduction of nitroaromatics compounds under visible-light irradiation
Ghalebin, Saeed Nasiri,Bezaatpour, Abolfazl,Sadr, Moayad Hossaini,Sadjadi, Mirabdullah Seyed,Moghaddam, Mohammad Khodadadi,Szunerits, Sabine
, (2021/01/26)
In this work, [1 + 1] macrocyclic Copper...
Gold supported on titania for specific monohydrogenation of dinitroaromatics in the liquid phase
Liu, Shuang-Shuang,Liu, Xiang,Yu, Lei,Liu, Yong-Mei,He, He-Yong,Cao, Yong
, p. 4162 - 4169 (2014/09/29)
Liquid-phase selective monohydrogenation...
A new class of heterogeneous platinum catalysts for the chemoselective hydrogenation of nitroarenes
Pandarus, Valerica,Ciriminna, Rosaria,Beland, Francois,Pagliaro, Mario
scheme or table, p. 1306 - 1316 (2011/06/25)
A new series of nanostructured platinum ...
Selective partial hydrogenation of dinitrobenzenes to nitroanilines catalyzed by Ru/C
Hou, Jie,Ma, Yonghuan,Li, Yuhan,Guo, Fang,Lu, Lianhai
scheme or table, p. 974 - 975 (2009/04/06)
Ru/C was found to be a highly effective ...
5307-14-2 Process route
-
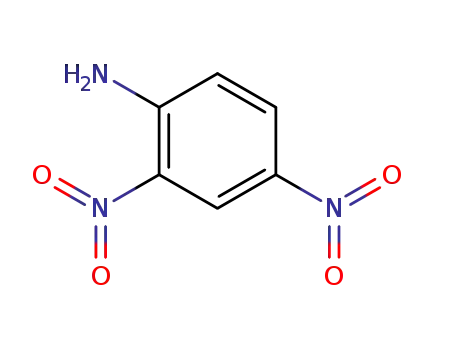
-
97-02-9
2,4-Dinitroanilin

-
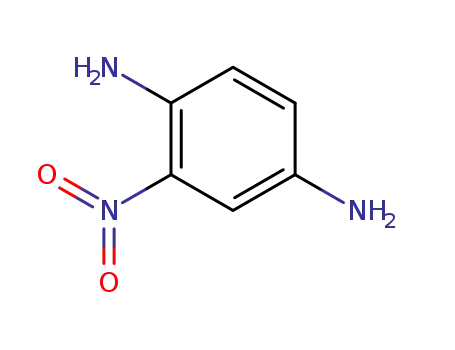
-
5307-14-2,183870-67-9
2-nitro-1,4-phenylenediamine
| Conditions | Yield |
|---|---|
|
With
sulfuric acid; hydrogen;
platinum on activated charcoal;
In
acetic acid;
at 85 ℃;
|
48% |
|
With
hydrazine hydrate;
In
methanol; dichloromethane; acetonitrile;
at 25 ℃;
Irradiation;
|
-

-
97-02-9
2,4-Dinitroanilin

-

-
5307-14-2,183870-67-9
2-nitro-1,4-phenylenediamine

-
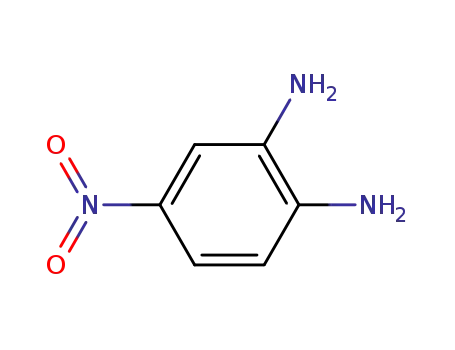
-
99-56-9
4-Nitrophenylene-1,2-diamine
| Conditions | Yield |
|---|---|
|
With
nickel; hydrazine hydrate;
In
ethanol; 1,2-dichloro-ethane;
at 50 - 60 ℃;
for 6h;
|
3% 95% |
|
With
ethanol; sodium hydrogensulfite;
|
|
|
With
ammonium sulfide;
|
|
|
With
sodium hydrogensulfide; ethanol;
|
|
|
With
ruthenium on carbon; hydrogen;
at 90 ℃;
under 11251.1 Torr;
|
|
|
With
hydrogen;
In
methanol;
at 20 ℃;
for 0.5h;
under 750.075 Torr;
chemoselective reaction;
|
|
|
With
hydrogen;
In
ethanol;
at 60 ℃;
for 9.5h;
under 22502.3 Torr;
regioselective reaction;
|
5307-14-2 Upstream products
-
7418-43-1
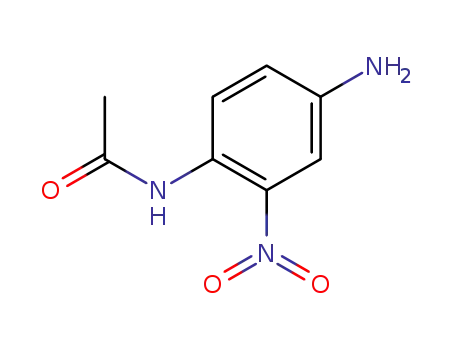
nitro-p-phenylenediamine N1-monoacetate
-
6086-29-9
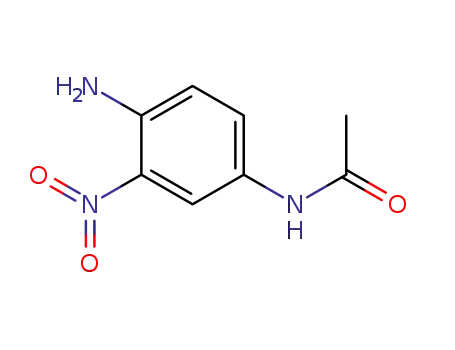
4-acetylamino-1-amino-2-nitrobenzene
-
5345-53-9
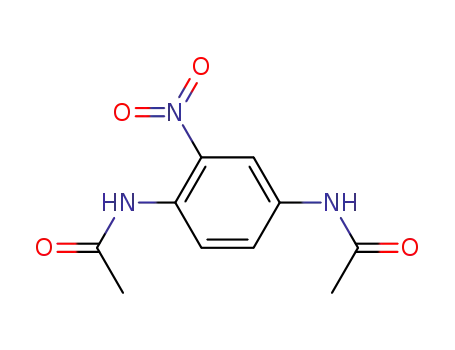
nitro-p-phenylenediamine N1,N4-diacetate
-
861532-87-8
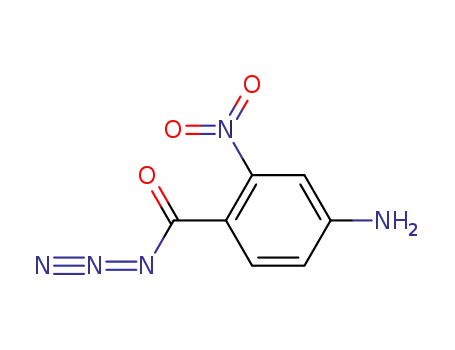
4-amino-2-nitro-benzoyl azide
5307-14-2 Downstream products
-
14990-16-0
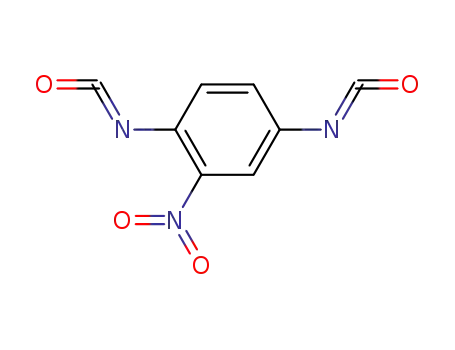
nitro-p-phenylene diisocyanate
-
6086-29-9

4-acetylamino-1-amino-2-nitrobenzene
-
27173-95-1
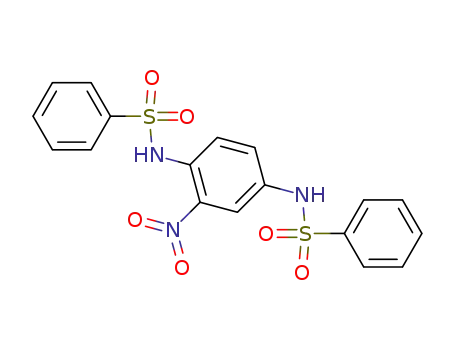
N,N'-(nitro-p-phenylene)-bis-benzenesulfonamide
-
73320-73-7
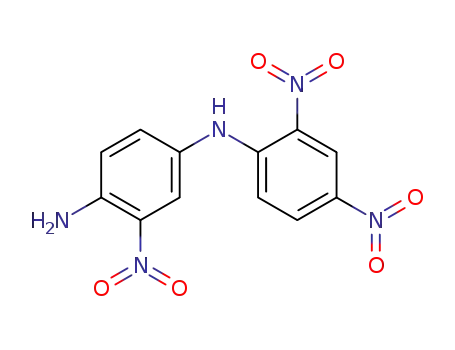
N4-(2,4-dinitro-phenyl)-2-nitro-p-phenylenediamine


