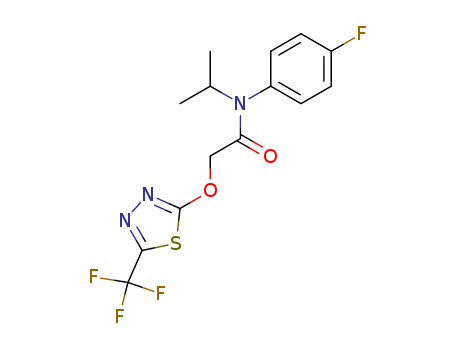
Flufenacet
-
Product Name :
Flufenacet
-
CAS No :
142459-58-3
-
Project State :
Commercial
Application
General Description
Good factory supply good Flufenacet 142459-58-3
- Molecular Formula:C14H13F4N3O2S
- Molecular Weight:363.336
- Vapor Pressure:1.18E-06mmHg at 25°C
- Melting Point:75-77oC
- Refractive Index:1.538
- Boiling Point:401.5 °C at 760mmHg
- PKA:0.31±0.50(Predicted)
- Flash Point:196.6 °C
- PSA:83.56000
- Density:1.416 g/cm3
- LogP:3.51640
Flufenacet(Cas 142459-58-3) Usage
|
Trade name |
AXIOM? Thiafluamide; DOMAIN?; EPIC?; FOE 5043? technical |
|
Definition |
ChEBI: An aromatic amide that is acetamide in which the amino hydrogens have been replaced by a propan-2-yl and 4-fluorophenyl groups while the methyl hydrogen is replaced by a [5-(trifluoromethyl)-1,3,4-thiadiazol-2-yl]oxy group. |
|
Agricultural Uses |
Herbicide: Flufenacet is applied to the soil surface or incorporated pre-emergence in field corn, corn grown for silage, or soybeans to control certain annual grasses and broadleaf weeds. Not listed for use in EU countries. Registered for use in the U.S. |
InChI:InChI=1/C14H13F4N3O2S/c1-8(2)21(10-5-3-9(15)4-6-10)11(22)7-23-13-20-19-12(24-13)14(16,17)18/h3-6,8H,7H2,1-2H3
142459-58-3 Relevant articles
HERBICIDAL COMPOSITIONS
-
, (2022/03/02)
The present invention provides compositi...
Flufenacet preparation method
-
Paragraph 0048-0050, (2018/04/03)
The invention discloses a flufenacet pre...
SYNTHESIS METHOD OF THIADIAZOLYLAMIDE DERIVATIVE
-
Paragraph 0023, (2015/12/08)
A synthesis method of 2-[(5-(trifluorome...
142459-58-3 Process route
-
-
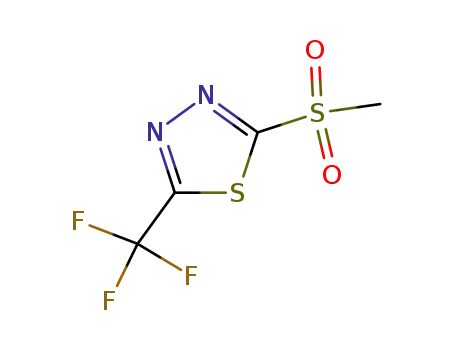
27603-25-4
2-(methylsulfonyl)-5-(trifluoromethyl)-1,3,4-thiadiazole

-
-
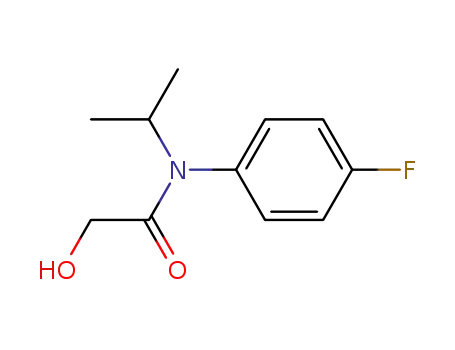
54041-17-7
N-(4-fluorophenyl)-2-hydroxy-N-(1-methylethyl)acetamide

-
-
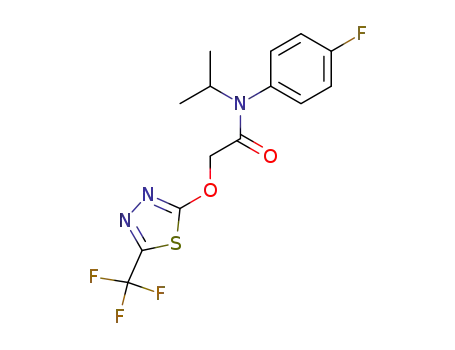
142459-58-3
flufenacet
| Conditions | Yield |
|---|---|
|
With
iron(II) chloride;
In
1,2-dichloro-ethane;
at 75 ℃;
for 2h;
|
98% |
|
With
sodium hydroxide;
In
water; acetone;
at -20 ℃;
for 3h;
|
-

-
54041-17-7
N-(4-fluorophenyl)-2-hydroxy-N-(1-methylethyl)acetamide

-
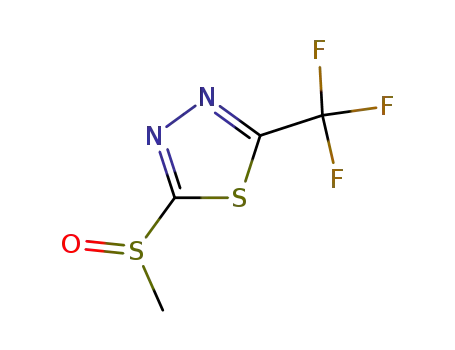
-
65439-30-7
2-methylsulphinyl-5-trifluoromethyl-1,3,4-thiadiazole

-

-
142459-58-3
flufenacet
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
In
dichloromethane; water;
at 5 ℃;
for 2h;
Solvent;
Reagent/catalyst;
|
98.5% |
142459-58-3 Upstream products
-
54041-17-7

N-(4-fluorophenyl)-2-hydroxy-N-(1-methylethyl)acetamide
-
65439-30-7

2-methylsulphinyl-5-trifluoromethyl-1,3,4-thiadiazole
-
66602-64-0
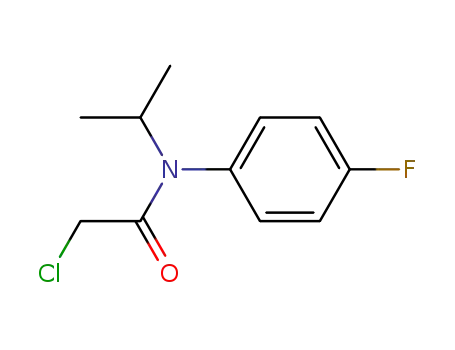
2-chloro-N-(4-fluorophenyl)-N-(1-methylethyl)acetamide
-
27603-25-4

2-(methylsulfonyl)-5-(trifluoromethyl)-1,3,4-thiadiazole


