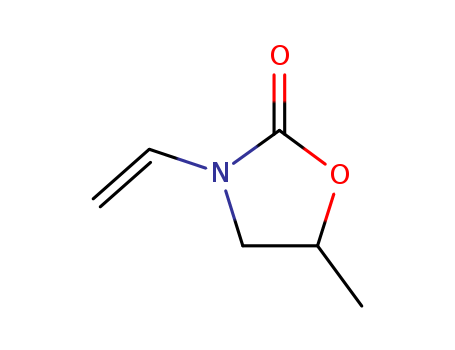
5-Methyl-3-vinyl-2-oxazolidinone
-
Product Name :
5-Methyl-3-vinyl-2-oxazolidinone
-
CAS No :
3395-98-0
-
Project State :
Commercial
Application
General Description
Cost-effective and customizable 5-Methyl-3-vinyl-2-oxazolidinone 3395-98-0 for sale
- Molecular Formula:C6H9NO2
- Molecular Weight:127.143
- Vapor Pressure:2.2-25Pa at 20-50℃
- Boiling Point:78-81 °C(Press: 1.3 Torr)
- PKA:-0.39±0.40(Predicted)
- PSA:29.54000
- Density:1.085 g/cm3
- LogP:0.90850
3395-98-0 Relevant articles
SYNTHESIS OF N-VINYL COMPOUNDS BY REACTING CYLIC NH-COMPOUNDS WITH ACETYLENE IN PRESENCE OF HOMOGENOUS CATALYST
-
Page/Page column 17; 21-22, (2021/06/26)
Process to produce N-vinyl compounds by ...
Phosphine-Catalyzed Vinylation at Low Acetylene Pressure
Sitte, Nikolai A.,Menche, Maximilian,Tu?ina, Pavel,Bienewald, Frank,Sch?fer, Ansgar,Comba, Peter,Rominger, Frank,Hashmi, A. Stephen K.,Schaub, Thomas
, p. 13041 - 13055 (2021/09/18)
The vinylation of various nucleophiles w...
Ruthenium-catalyzed synthesis of vinylamides at low acetylene pressure
Semina, Elena,Tuzina, Pavel,Bienewald, Frank,Hashmi,Schaub, Thomas
supporting information, p. 5977 - 5980 (2020/06/04)
The reaction of cyclic amides with acety...
Process for production of N-vinyl compound
-
, (2008/06/13)
The present invention provides a process...
3395-98-0 Process route
-
-
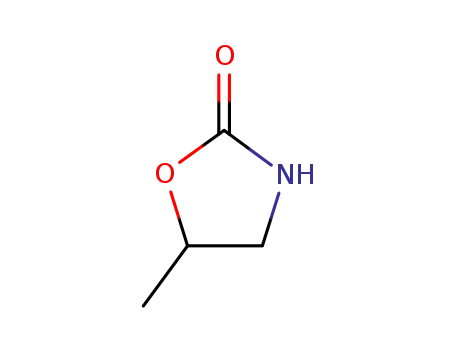
1072-70-4
5-methyl-1,3-oxazolidin-2-one

-
-
74-86-2,25067-58-7
acetylene

-
-
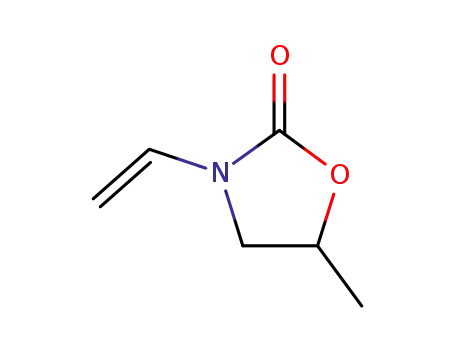
3395-98-0
5-methyl-3-vinyloxazolidin-2-one
| Conditions | Yield |
|---|---|
|
With
[bis(2-methylallyl)cycloocta-1,5-diene]ruthenium(II); tributylphosphine;
In
toluene;
at 100 ℃;
for 16h;
Reagent/catalyst;
Autoclave;
Glovebox;
|
84% |
|
With
tributylphosphine;
In
toluene;
at 100 ℃;
for 16h;
Reagent/catalyst;
Autoclave;
Inert atmosphere;
Glovebox;
|
84% |
|
With
tributylphosphine;
In
N,N-dimethyl acetamide;
at 140 ℃;
for 16h;
under 1.5 Torr;
Glovebox;
Sealed tube;
Autoclave;
|
52% |
-
-
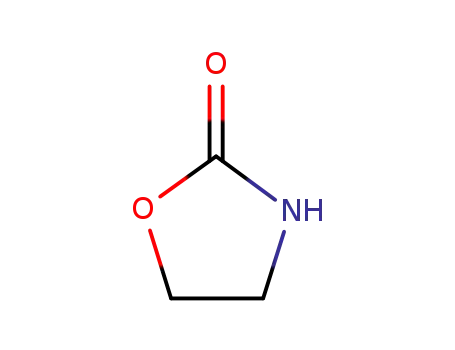
497-25-6
dimethylenecyclourethane

-
-
74-86-2,25067-58-7
acetylene

-

-
3395-98-0
5-methyl-3-vinyloxazolidin-2-one
| Conditions | Yield |
|---|---|
|
With
tributylphosphine;
In
toluene;
at 100 ℃;
for 16h;
Reagent/catalyst;
Autoclave;
Inert atmosphere;
Glovebox;
|
29% |
3395-98-0 Upstream products
-
123403-99-6
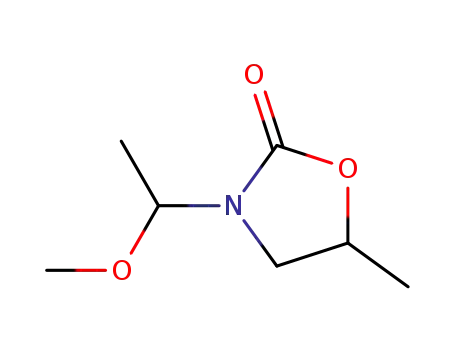
N-(1-methoxyethyl)-5-methyl-2-oxazolidone
-
123404-00-2
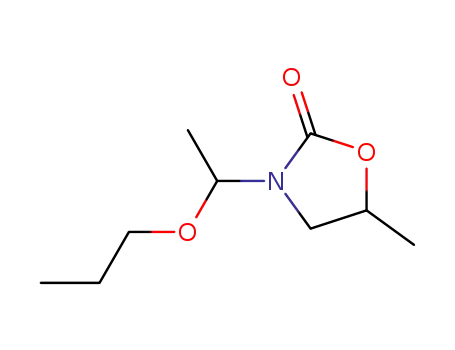
N-(1-propoxyethyl)-5-methyl-2-oxazolidone
-
1072-70-4

5-methyl-1,3-oxazolidin-2-one
-
74-86-2
acetylene
3395-98-0 Downstream products
-
7594-64-1
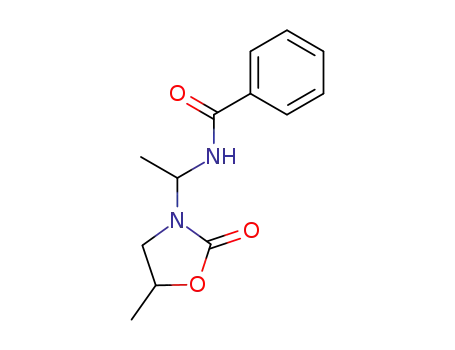
N-<1-Benzamino-aethyl>-5-methyl-2-oxo-oxazolidin
-
7594-63-0
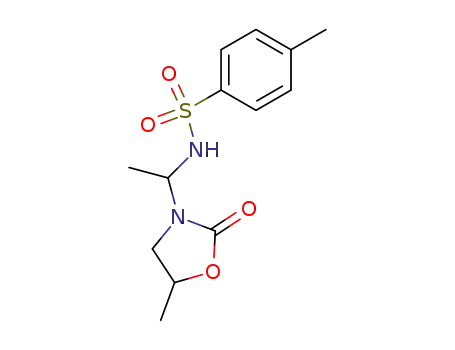
5-methyl-3-[1-(toluene-4-sulfonylamino)-ethyl]-oxazolidin-2-one
-
5681-86-7
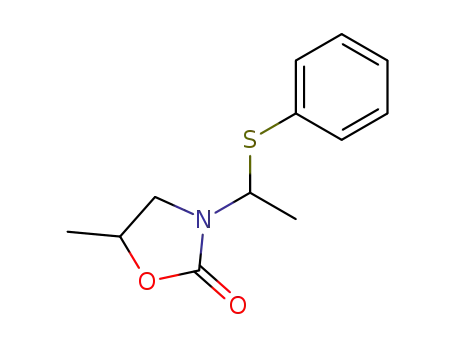
5-methyl-3-(1-phenylsulfanyl-ethyl)-oxazolidin-2-one
-
7594-62-9
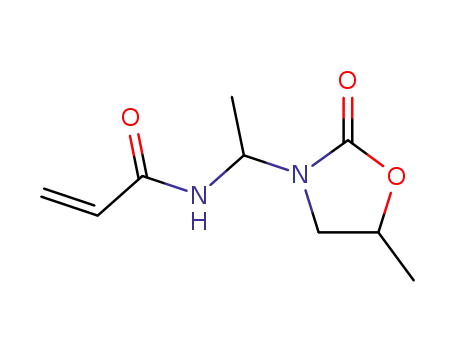
N-<1-Acryloylamino-aethyl>-5-methyl-2-oxo-oxazolidin


