9,9'-BIFLUORENYLIDENE
-
Product Name :
9,9'-BIFLUORENYLIDENE
-
CAS No :
746-47-4
-
Project State :
Commercial
Application
General Description
Buy cost-effective 99% pure 9,9'-BIFLUORENYLIDENE 746-47-4 now
- Molecular Formula:C26H16
- Molecular Weight:328.413
- Vapor Pressure:1.23E-09mmHg at 25°C
- Melting Point:187 °C
- Refractive Index:1.8430 (estimate)
- Boiling Point:499.9°C at 760 mmHg
- Flash Point:252.4°C
- PSA:0.00000
- Density:1.265g/cm3
- LogP:6.65480
9,9'-BIFLUORENYLIDENE(Cas 746-47-4) Usage
|
General Description |
9,9'-BIFLUORENYLIDENE, also known as 9,9'-BIFLUORENE, is a chemical compound with the molecular formula C26H14. It is a highly aromatic and stable molecule consisting of two fluorene units connected by a carbon-carbon double bond. 9,9'-BIFLUORENYLIDENE is widely used as a building block in the synthesis of various organic materials and functional molecules, including organic light emitting diodes (OLEDs), organic semiconductors, and photosensitizers. 9,9'-BIFLUORENYLIDENE exhibits strong fluorescence and is known for its thermally stable and optically active properties, making it a valuable component in the development of advanced organic electronic and photonic devices. Furthermore, its unique structure and reactivity make it a versatile precursor for the construction of novel organic materials with potential applications in optoelectronic devices and advanced materials science. |
InChI:InChI=1/C26H16/c1-5-13-21-17(9-1)18-10-2-6-14-22(18)25(21)26-23-15-7-3-11-19(23)20-12-4-8-16-24(20)26/h1-16H
746-47-4 Relevant articles
-
Yano et al.
, p. 2739 (1979)
-
-
Campaigne,Reid
, p. 769 (1946)
-
REACTIONS OF 1-OXA-3-AZONIABUTATRIENE SALTS WITH 1,3-DIPOLES
Hamed, Atef,MueLler, Edgar,Jochims, Johannes C.,Zsolnai, Laszlo,Huttner, Gottfried
, p. 5825 - 5836 (1989)
The nitrile N-oxide 4 undergoes a revers...
Regioselectivity of the 1,3-dipolar cycloaddition of fluorinated fluoren-9-iminium ylides to heteroelement-containing dipolarophiles: Experimental and quantum-chemical study
Novikov,Khlebnikov,Egarmin,Shevchenko,Khlebnikov,Kostikov,Vidovic
, p. 1800 - 1812 (2006)
N-Substituted 9H-fluoren-9-imines react ...
Structural influences impacting the role of the 9-ylidene bond in the electronic tuning of structures built upon 9-fluorenylidene scaffolds
Eakins, Galen L.,Cooper, Matthew W.,Gerasimchuk, Nikolay N.,Phillips, Terry J.,Breyfogle, Bryan E.,Stearman, Chad J.
, p. 1059 - 1071 (2013)
A structure-effect study is presented pe...
1,3-Dipolar cycloadditions, 97: Some cycloadditions of aromatic thione S-oxides
Huisgen, Rolf,Mloston, Grzegorz,Polborn, Kurt,Palacios-Gambra, Francisco
, p. 187 - 192 (1997)
Surprisingly, thiobenzophenone S-oxide (...
The reaction of electrophilic terminal phosphinidene complexes with benzophenone and fluorenone: The unexpected formation of six- and eight-membered heterocycles
Inubushi, Yoichi,Huy, Ngoc Hoa Tran,Ricard, Louis,Mathey, Francois
, p. 83 - 86 (1997)
The phenylphosphinidene-pentacarbonyltun...
A Kinetic Study on Denitration of 9,9'-Dinitro-9,9'-bifluorenyl by Tin(II) Chloride in N,N-Dimethylformamide
Fukunaga, Kimitoshi,Kimura, Makoto
, p. 3191 - 3192 (1983)
The kinetics of the reductive eliminatio...
Trivalent organophosphorus reagent induced pinacol rearrangement of 4H-cyclopenta[2,1-b:3,4-b′]dithiophen-4-one
Marin, Lidia,Zhang, Yuexing,Robeyns, Koen,Champagne, Beno?t,Adriaensens, Peter,Lutsen, Laurence,Vanderzande, Dirk,Bevk, David,Maes, Wouter
, p. 526 - 529 (2013)
In this Letter we report on the reaction...
TRANSITION METAL PROMOTED REACTIONS XIV. NOVEL REDUCTIVE COUPLING OF THIOKETALS WITH MOLYBDENUM HEXACARBONYL
Wong, Chi Sang,Leung, Wing Sang,Yeung, Lam Lung,Luh, Tien-Yau
, p. C49 - C51 (1986)
Thioketals of fluorenone, acetophenone, ...
Inclusion crystals of 2,2′,7,7′,9,9′-hexahalo-9,9′-bisfluorenyl derivatives: a new family of polyhalo aryl hosts
Tanaka, Koichi,Wada, Shin-ichi,Caira, Mino R.
, p. 9213 - 9220 (2007)
The preparation and inclusion properties...
(PNP)Cobalt-Catalyzed Olefination of Diazoalkanes
Chirik, Paul J.,Semproni, Scott P.,Yruegas, Sam
supporting information, (2022/03/17)
Addition of excess diazoalkane to the pi...
α-Bromoacrylic Acids as C1 Insertion Units for Palladium-Catalyzed Decarboxylative Synthesis of Diverse Dibenzofulvenes
Zhang, Minghao,Deng, Wenbo,Sun, Mingjie,Zhou, Liwei,Deng, Guobo,Liang, Yun,Yang, Yuan
, p. 5744 - 5749 (2021/08/18)
Herein α-bromoacrylic acids have been em...
Direct Observation of Aggregation-Induced Emission Mechanism
Corminboeuf, Clémence,Guan, Jianxin,Han, Han,Lin, Kun-Han,Liu, Jitian,Peng, Jie,Prlj, Antonio,Wei, Rong,Yu, Zhihao,Zhao, Dahui,Zheng, Junrong
supporting information, p. 14903 - 14909 (2020/07/04)
The mechanism of aggregation-induced emi...
Substituent Effects on Reactions of [RhCl(COD)]2 with Diazoalkanes
Cui, Mingxu,Lin, Shujuan,Sung, Herman H. Y.,Williams, Ian D.,Lin, Zhenyang,Jia, Guochen
, p. 905 - 915 (2019/03/04)
The reactions of [RhCl(COD)]2 with a ser...
746-47-4 Process route
-
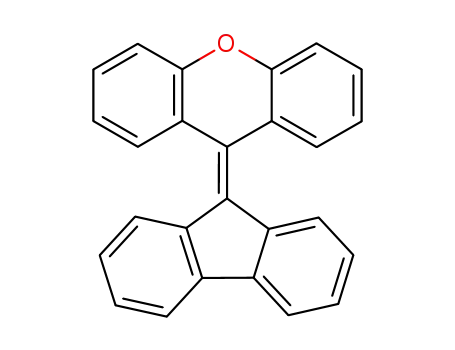
-
27090-15-9
9-(9'H-fluoren-9'-ylidene)-9H-xanthene

-
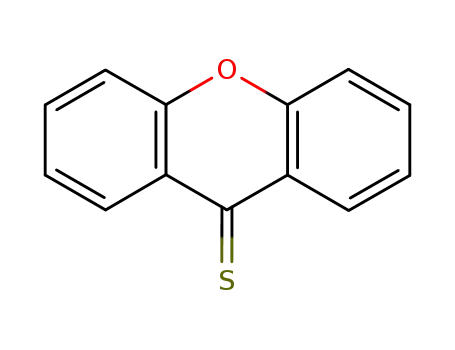
-
492-21-7
9H-xanthene-9-thione

-
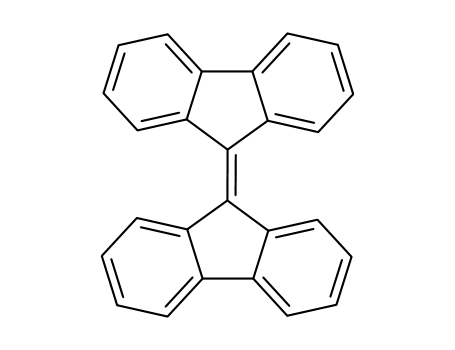
![tetrabenzo[5.5]fulvalene](/upload/2025/9/b55cf175-8786-48fb-9310-e5002c46a533.png)
-
746-47-4
tetrabenzo[5.5]fulvalene

-
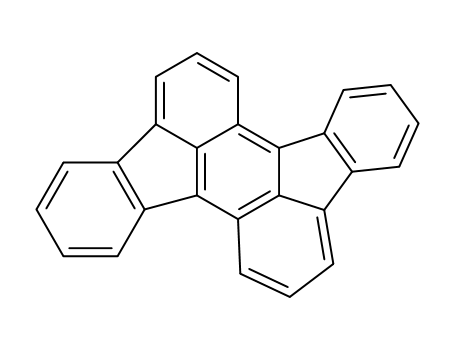
-
197-61-5
rubicene
| Conditions | Yield |
|---|---|
|
With
sulfur;
at 270 - 280 ℃;
for 0.0833333h;
|
38% 1.3% 45% |
-
-
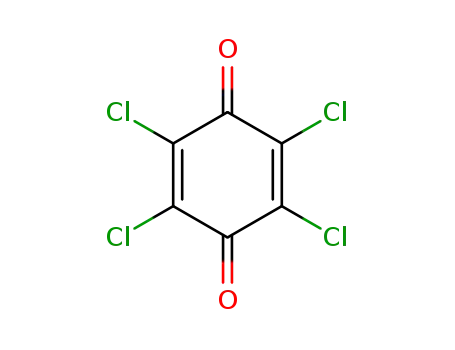
118-75-2
chloranil

-
-
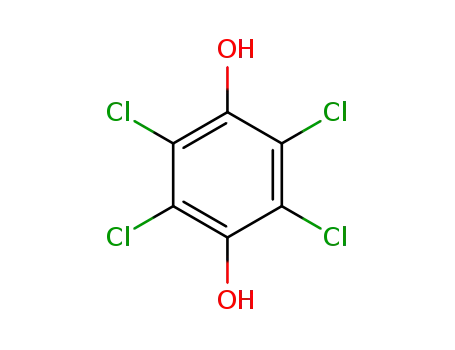
87-87-6
2,3,5,6-tetrachlorobenzene-1,4-diol

-
![tetrabenzo[5.5]fulvalene](/upload/2025/9/b55cf175-8786-48fb-9310-e5002c46a533.png)
-
746-47-4
tetrabenzo[5.5]fulvalene

-
-
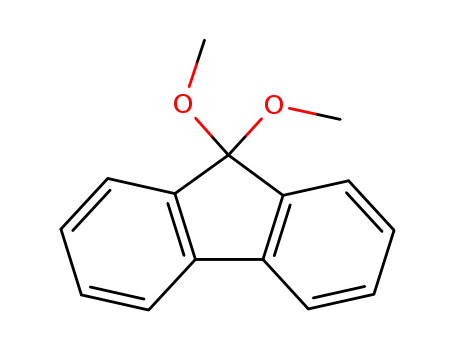
116143-54-5
9-fluorenone dimethyl acetal
| Conditions | Yield |
|---|---|
|
With
methanol; 9-diazofluorenone;
In
benzene;
for 10h;
Heating;
|
90% 7% 5% |
746-47-4 Upstream products
-
109-99-9
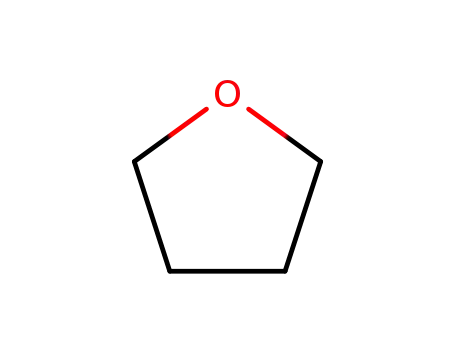
tetrahydrofuran
-
1940-57-4
9H-fluoren-9-yl bromide
-
100-67-4
potassium phenolate
-
60-29-7
diethyl ether
746-47-4 Downstream products
-
486-25-9
9-fluorenone
-
1530-12-7
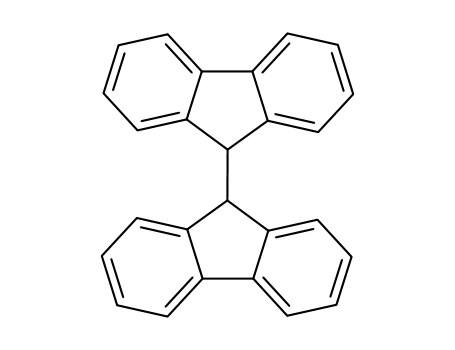
9,9'-bifluorenyl
-
103389-40-8
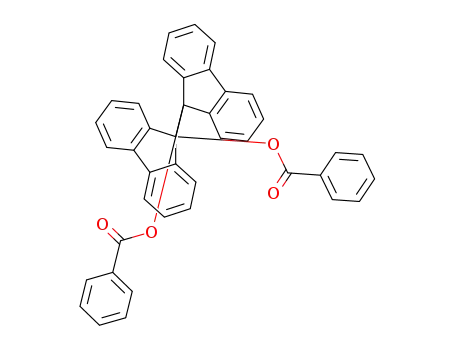
9,9'-bis-benzoyloxy-[9,9']bifluorenyl
-
86-73-7
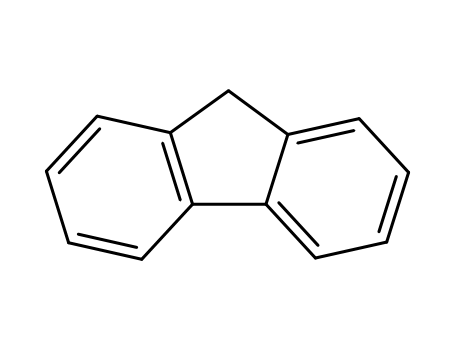
9H-fluorene


