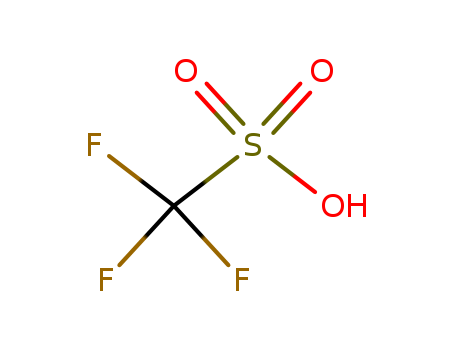
Trifluoromethanesulfonic acid
-
Product Name :
Trifluoromethanesulfonic acid
-
CAS No :
1493-13-6
-
Project State :
Commercial
Application
General Description
Buy cost-effective 99% pure Trifluoromethanesulfonic acid 1493-13-6 now
- Molecular Formula:CHF3O3S
- Molecular Weight:150.078
- Appearance/Colour:Colourless liquid with a pungent odour
- Vapor Pressure:8 mm Hg ( 25 °C)
- Melting Point:-40 °C
- Refractive Index:n20/D 1.327(lit.)
- Boiling Point:161.999 °C at 760 mmHg
- PKA:-14(at 25℃)
- Flash Point:None
- PSA:62.75000
- Density:1.877 g/cm3
- LogP:1.47480
Trifluoromethanesulfonic acid(Cas 1493-13-6) Usage
|
Chemical Description |
Trifluoromethanesulfonic acid is an organic compound with the formula CF3SO3H. |
|
Preparation |
Yellow-brown liquid. The boiling point is 167~170 ℃.The refractive index is 1.331.The relative density is 1.708.It is the strongest organic acids, easily soluble in water.Use carbon disulfide as raw material, with the reaction of iodine pentafluoride to produce trifluoromethyl disulfide.(CF3S) 2Hg was obtained when reacting with mercury; Then through oxidation of hydrogen oxide, trifluoromethanesulfonic acid is acquired. |
|
Reactions |
Trifluoromethanesulfonic acid acts as a catalyst for esterification reactions and an acidic titrant in nonaqueous acid-base titration. It is useful in protonations due to the presence of conjugate base triflate is non nucleophilic. It serves as a deglycosylation agent for glycoproteins. In addition, it is a precursor and a catalyst in organic chemistry. It reacts with acyl halides to prepare mixed triflate anhydrides, which are strong acylating agents used in Friedel-Crafts reactions. It acts as a key starting material for the preparation of ethers and olefins by reacting with alcohols as well as to prepare trifluoromethanesulfonic anhydride by dehydration reaction. Catalyst used in the production of cocoa butter substitute from palm oil. This is a very similar reaction to what would be done if one wanted to create polymers using triflic acid in the synthesis. Other Friedel-Crafts type reactions using triflic acid include cracking of alkanes and alkylation of alkenes which are very important to the petroleum industry. These triflic acid derivative catalysts are very effective in isomerizing straight chain or slightly branched hydrocarbons that can increase the octane rating of a particular petroleum based fuel. |
|
Definition |
ChEBI: Trifluoromethanesulfonic acid is a one-carbon compound that is methanesulfonic acid in which the hydrogens attached to the methyl carbon have been replaced by fluorines. It is a one-carbon compound and a perfluoroalkanesulfonic acid. It is a conjugate acid of a triflate. |
|
General Description |
Trifluoromethanesulfonic acid is a strong organic acid. It can be prepared by reacting bis(trifluoromethylthio)mercury with H2O2. On mixing with HNO3, it affords a nitrating reagent (a nitronium salt). This reagent is useful for the nitration of aromatic compounds. Its dissociation in various organic solvents has been studied. |
|
Safety Profile |
A corrosive irritant to the skin, eyes, and mucous membranes. A strong acid. Violent reaction with acyl chlorides or aromatic hydrocarbons evolves toxic hydrogen chloride gas. When heated to decomposition it emits toxic fumes of Fand SOx. See also FLUORIDES. |
InChI:InChI=1/CHF3.H2O3S/c2-1(3)4;1-4(2)3/h1H;4H,(H,1,2,3)
1493-13-6 Relevant articles
REACTIVITE DES ESTERS SULFONIQUES ET PYROSULFONIQUES PERFLUORES RFSO3RF ET RFSO3SO3RF. HETEROLYSE DE LA LIAION S-O
Oudrhiri-Hassani, M.,Brunel, D.,Germain, A
, p. 163 - 178 (1986)
Perfluoroalkyl perfluoroalkanesulfonates...
The vibrational spectrum of trifluoromethanesulphonic acid, CF3SO3H, and the determination of its degrees of discociation in aqueous solution by Raman spectroscopy
Edwards, H. G. M.
, p. 715 - 720 (1989)
The Raman and i.r. spectra of trifluorom...
Perfluoroalkanesulfonylimids and their lithium salts: Synthesis and characterisation of intermediates and target compounds
Conte, Lino,Gambaretto, Gian Paolo,Caporiccio, Gerardo,Alessandrini, Fabrizio,Passerini, Stefano
, p. 243 - 252 (2004)
ECF processes have been extensively expe...
Thermodynamics of methanesulfonic acid revisited
Guthrie,Gallant
, p. 1295 - 1298 (2000)
Recently we reported a study of the ther...
Photoinduced and Thermally Induced Rearrangements in a Thianthrenium Salt System
Saeva, Franklin D.
, p. 37 - 39 (1987)
A low-lying ?* level localized on a sulp...
SYNTHESIS OF TRIFLUOROMETHANESULFONATE ESTERS BY REATION OF ALKYL CHLORIDES WITH CHLORINE (I) AND BROMINE (I) TRIFLUOROMETHANESULFONATE
Katsuhara,Yutaka,Desmarteau, Darryl D.
, p. 257 - 264 (1980)
The synthesis of the first trifluorometh...
Remarkable Acid Catalysis in Proton-Coupled Electron-Transfer Reactions of a Chromium(III)-Superoxo Complex
Devi, Tarali,Lee, Yong-Min,Nam, Wonwoo,Fukuzumi, Shunichi
, p. 8372 - 8375 (2018)
Much enhanced acid catalysis was observe...
-
Rapp et al.
, p. 3642,3644 (1950)
-
Synthesis of trifluoromethanesulfonic acid from CHF3
Mukhopadhyay, Sudip,Bell, Alexis T.,Vijay Srinivas,Smith, Gary S.
, p. 660 - 662 (2004)
Trifluoromethane is transformed to trifl...
METHOD FOR PREPARING OXYSULPHIDE AND FLUORINATED DERIVATIVES IN THE PRESENCE OF AN ORGANIC SOLVENT
-
Paragraph 0193-0195, (2019/04/05)
The present invention concerns a method ...
PROCESS FOR THE PREPARATION OF HALOALKANESULFONIC ACIDS FROM SULFUR TRIOXIDE AND A HALOALKANE
-
Page/Page column 16; 17, (2019/12/15)
The present invention relates to a proce...
Water determines the products: An unexpected Br?nsted acid-catalyzed PO-R cleavage of P(iii) esters selectively producing P(O)-H and P(O)-R compounds
Li, Chunya,Wang, Qi,Zhang, Jian-Qiu,Ye, Jingjing,Xie, Ju,Xu, Qing,Han, Li-Biao
supporting information, p. 2916 - 2922 (2019/06/18)
Water is found able to determine the sel...
1493-13-6 Process route
-
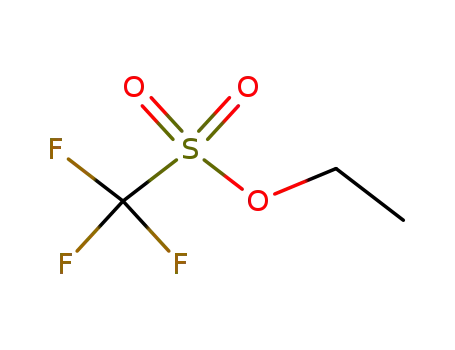
-
425-75-2
trifluoromethanesulfonic acid ethyl ester

-
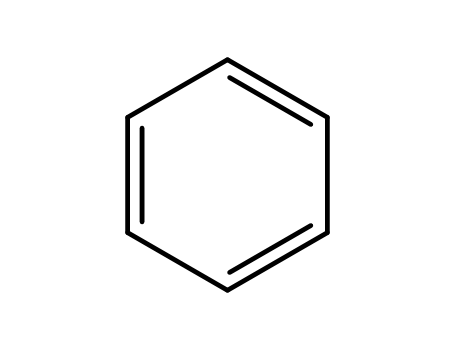
-
71-43-2,26181-88-4,54682-86-9,13967-78-7,174973-66-1
benzene

-

-
1493-13-6
trifluorormethanesulfonic acid

-
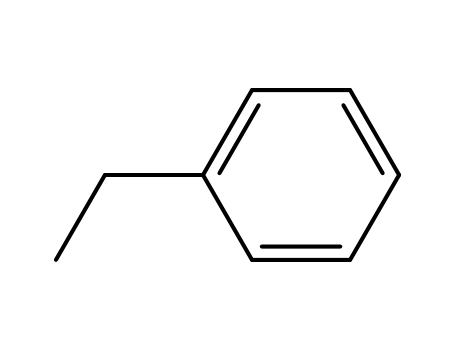
-
100-41-4,27536-89-6
ethylbenzene

-
-
253185-02-3,135-01-3
ortho-diethylbenzene

-
-
781-43-1
9,10-dimethylanthracene
| Conditions | Yield |
|---|---|
|
|
-
-
67-56-1
methanol

-
-
154318-75-9
4-tert-butylphenyl triflate

-
-
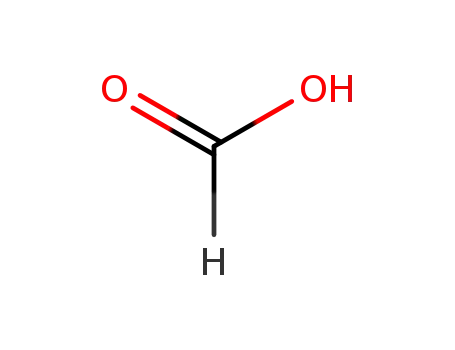
64-18-6
formic acid

-
-
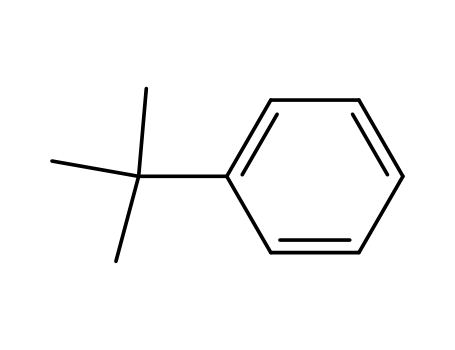
253185-03-4,253185-04-5
tert-butylbenzene

-
-
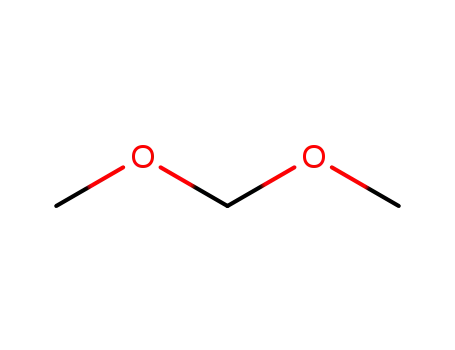
109-87-5
Dimethoxymethane

-
-
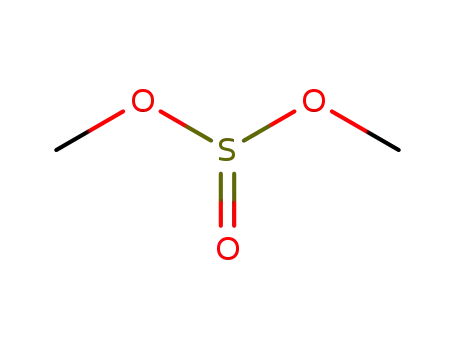
616-42-2
dimethylsulfite

-
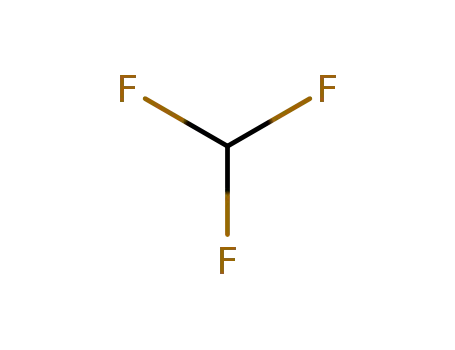
-
75-46-7
trifluoromethan

-

-
1493-13-6
trifluorormethanesulfonic acid

-
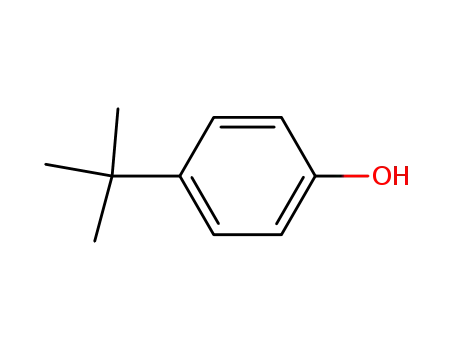
-
98-54-4
para-tert-butylphenol

-
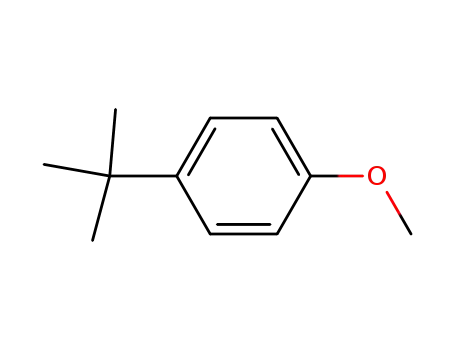
-
5396-38-3
1-(tert-butyl)-4-methoxybenzene

-
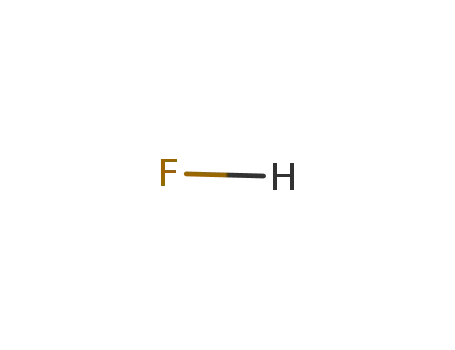
-
7664-39-3,12381-92-9,32057-09-3,74835-82-8
hydrogen fluoride

-
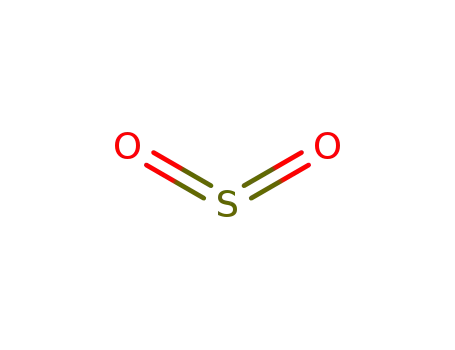
-
7446-09-5,12143-17-8,89125-89-3
sulfur dioxide
| Conditions | Yield |
|---|---|
|
With
oxygen;
for 6h;
Reagent/catalyst;
Mechanism;
Quantum yield;
Photolysis;
|
5 %Chromat. 60 %Chromat. |
1493-13-6 Upstream products
-
425-75-2

trifluoromethanesulfonic acid ethyl ester
-
335-05-7
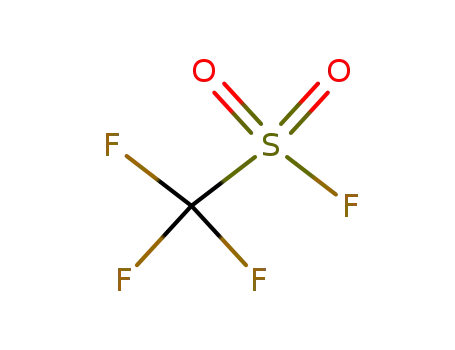
Trifluoromethanesulfonyl fluoride
-
60-29-7
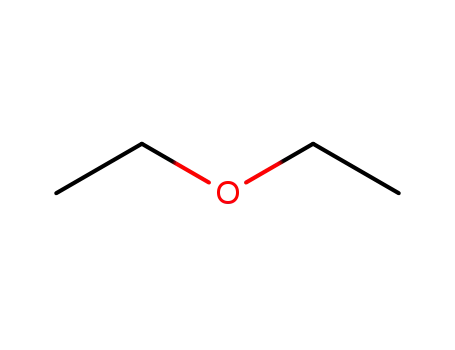
diethyl ether
-
333-27-7
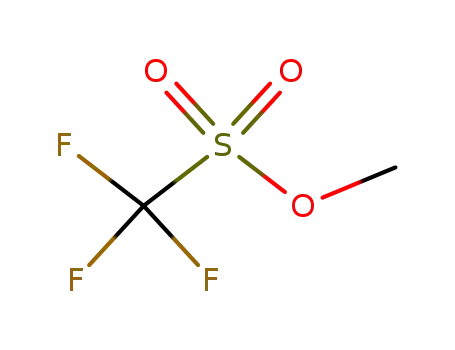
methyl trifluoromethanesulfonate
1493-13-6 Downstream products
-
421-83-0
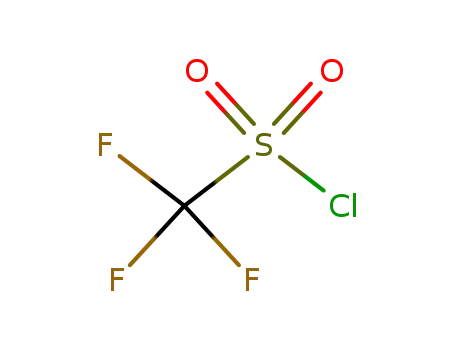
trifluoromethane sulfonyl chloride
-
358-23-6
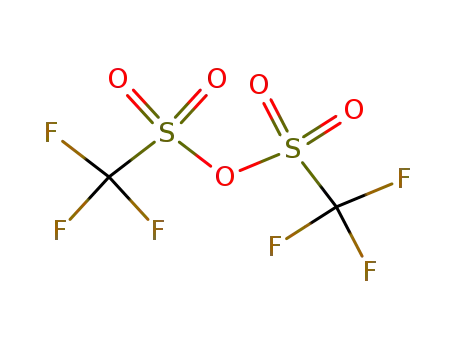
trifluoromethylsulfonic anhydride
-
61836-02-0

ethoxycarbonylmethyl trifluoromethanesulfonate
-
425-75-2

trifluoromethanesulfonic acid ethyl ester


