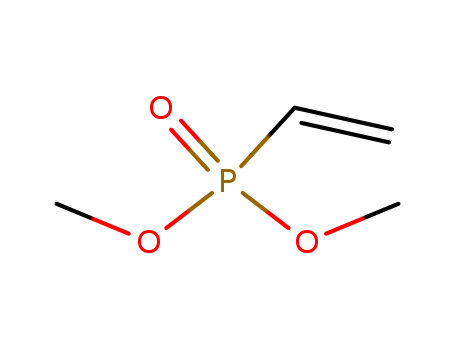
DIMETHYL VINYLPHOSPHONATE
-
Product Name :
DIMETHYL VINYLPHOSPHONATE
-
CAS No :
4645-32-3
-
Project State :
Commercial
Application
General Description
Cost-effective and customizable DIMETHYL VINYLPHOSPHONATE 4645-32-3 supplier
- Molecular Formula:C4H9 O3 P
- Molecular Weight:136.087
- Vapor Pressure:3.83mmHg at 25°C
- Melting Point:<-30°C
- Refractive Index:n20/D 1.428
- Boiling Point:197-202 °C(lit.)
- Flash Point:101°C
- PSA:45.34000
- Density:1.146 g/mL at 20 °C(lit.)
- LogP:1.61580
DIMETHYL VINYLPHOSPHONATE(Cas 4645-32-3) Usage
|
General Description |
Dimethyl vinylphosphonate is a chemical compound with the formula C5H9O3P, with a molecular weight of 146.09 g/mol. It is a colorless liquid with a fruity odor and is highly flammable. Dimethyl vinylphosphonate is commonly used as a monomer in the production of polymers and copolymers for various applications, including flame retardants, adhesives, and coatings. It is also used in the synthesis of pharmaceuticals, agrochemicals, and other specialty chemicals. Furthermore, dimethyl vinylphosphonate is known to react readily with nucleophiles, such as amines, alcohols, and thiols, making it a versatile building block in organic synthesis. However, it is important to handle dimethyl vinylphosphonate with care, as it is toxic and can cause skin and eye irritation upon contact. |
InChI:InChI=1/C4H9O3P/c1-4-8(5,6-2)7-3/h4H,1H2,2-3H3
4645-32-3 Relevant articles
-
Callot,H.J.,Benezra,C.
, p. 3382 - 3387 (1970)
-
METHOD FOR PRODUCING ALKENYLPHOSPHORUS COMPOUND
-
Paragraph 0068-0074, (2021/06/04)
PROBLEM TO BE SOLVED: To provide a metho...
Method for synthesizing allyl phosphonic acid derivative (by machine translation)
-
Paragraph 0041-0145, (2020/05/14)
The invention mainly solves the problems...
METHOD FOR PRODUCING ALKENYL PHOSPHORUS COMPOUND
-
Paragraph 0122-0166, (2019/09/06)
Provided is a method for producing an al...
PROCESS FOR PREPARING AN ALKENYLPHOSPHONIC ACID DERIVATIVE
-
Page/Page column 6, (2010/06/11)
Process for preparing an alkenylphosphon...
4645-32-3 Process route
-
-
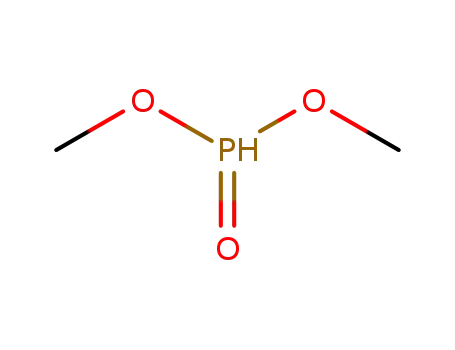
868-85-9
Dimethyl phosphite

-
-
74-86-2,25067-58-7
acetylene

-
-
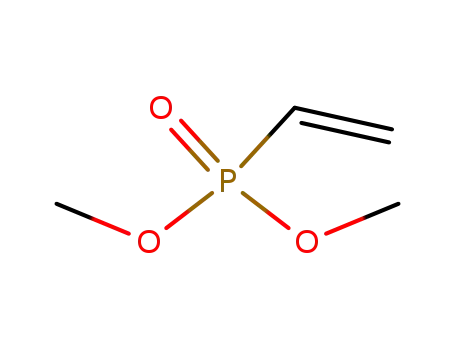
4645-32-3
dimethylvinylphosphonate
| Conditions | Yield |
|---|---|
|
Dimethyl phosphite;
With
1,8-diazabicyclo[5.4.0]undec-7-ene;
1,3-bis-(diphenylphosphino)propane; nickel(II) acetate tetrahydrate;
at 95 ℃;
for 0.166667h;
acetylene;
at 100 ℃;
for 1.5h;
under 760.051 Torr;
Product distribution / selectivity;
|
87% |
|
bis(acetylacetonate)nickel(II); 1,3-bis-(diphenylphosphino)propane; triphenylphosphine;
In
tetraethyleneglycol-dimethylether;
at 100 ℃;
for 1.66667h;
under 760.051 Torr;
Product distribution / selectivity;
|
80% |
|
bis(acetylacetonate)nickel(II); 1,3-bis-(diphenylphosphino)propane;
In
tetraethyleneglycoldimethylether;
at 100 ℃;
for 1.66667h;
Product distribution / selectivity;
|
70% |
|
With
cerium(IV) oxide; palladium(II) nitrate dihydrate; nitric acid;
In
tetrahydrofuran; water;
at 120 ℃;
for 4h;
under 15001.5 Torr;
Reagent/catalyst;
Temperature;
Pressure;
Autoclave;
Inert atmosphere;
|
53% |
|
With
phosphonic Acid; tetrakis(trimethylphosphine)nickel(0);
In
toluene;
at 0 ℃;
for 5h;
under 150.015 Torr;
Reagent/catalyst;
Concentration;
Pressure;
Temperature;
Catalytic behavior;
Autoclave;
|
-
-
74-86-2,25067-58-7
acetylene

-

-
4645-32-3
dimethylvinylphosphonate
| Conditions | Yield |
|---|---|
|
In
n-octyne;
|
92% |
|
In
n-octyne;
|
85% |
4645-32-3 Upstream products
-
67-56-1
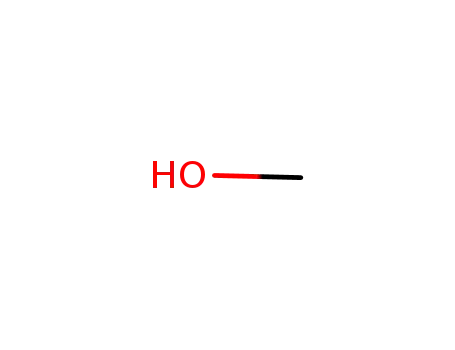
methanol
-
1438-74-0
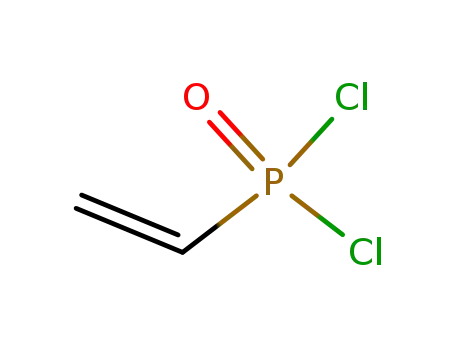
vinyldichlorophosphine oxide
-
26119-41-5
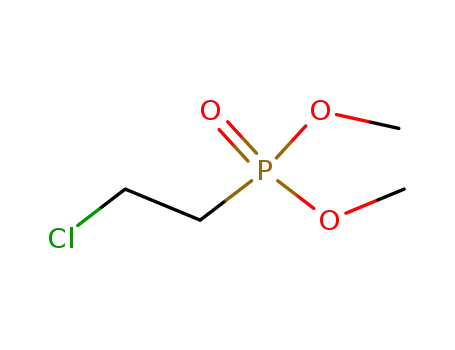
dimethyl 2-chloroethylphosphonate
-
106-93-4
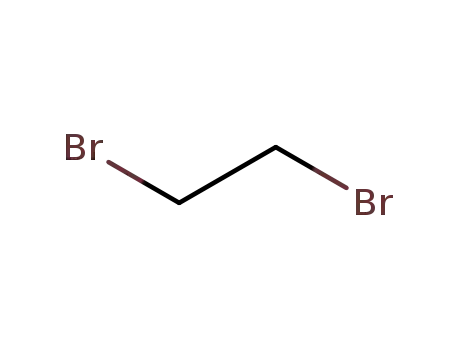
ethylene dibromide
4645-32-3 Downstream products
-
88691-17-2
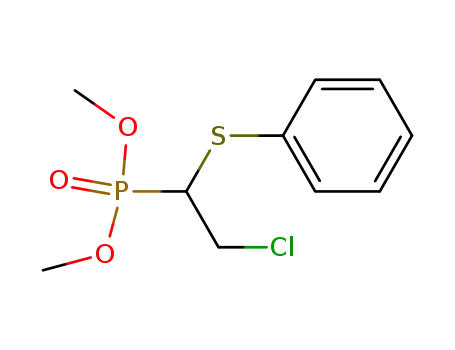
(2-Chloro-1-phenylsulfanyl-ethyl)-phosphonic acid dimethyl ester
-
185897-11-4
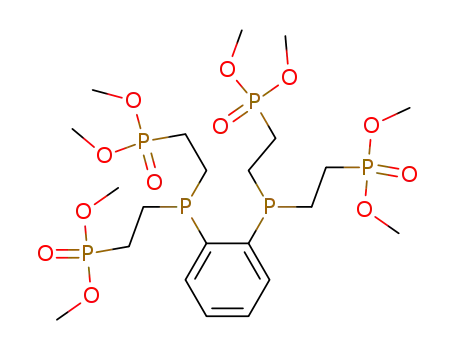
(2-{(2-{Bis-[2-(dimethoxy-phosphoryl)-ethyl]-phosphanyl}-phenyl)-[2-(dimethoxy-phosphoryl)-ethyl]-phosphanyl}-ethyl)-phosphonic acid dimethyl ester
-
131486-53-8
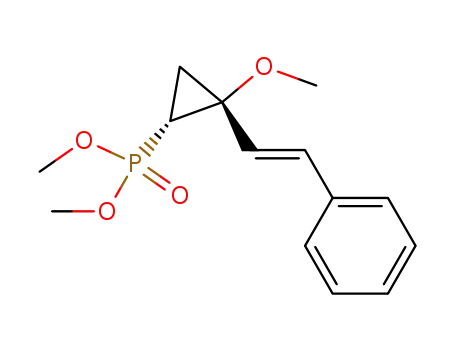
cis-<2-Methoxy-2-(2-phenylethenyl)cyclopropyl>phosphonsaeure-dimethylester
-
131407-55-1
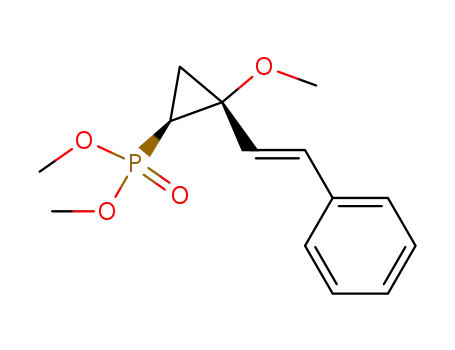
trans-<2-Methoxy-2-(2-phenylethenyl)cyclopropyl>phosphonsaeure-dimethylester


