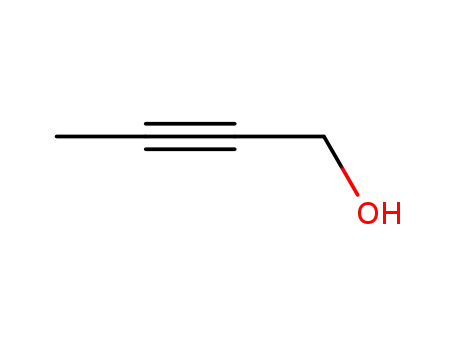
2-Butyn-1-ol
-
Product Name :
2-Butyn-1-ol
-
CAS No :
764-01-2
-
Project State :
Commercial

Application
General Description
Cost-effective and customizable 2-Butyn-1-ol 764-01-2 in stock
- Molecular Formula:C4H6O
- Molecular Weight:70.091
- Appearance/Colour:clear colourless to light yellow liquid
- Vapor Pressure:1.67mmHg at 25°C
- Melting Point:- 2.2 °C(lit.)
- Refractive Index:n20/D 1.453(lit.)
- Boiling Point:148 °C at 760 mmHg
- PKA:13.14±0.10(Predicted)
- Flash Point:51.7 °C
- PSA:20.23000
- Density:0.929 g/cm3
- LogP:0.00200
2-Butyn-1-ol(Cas 764-01-2) Usage
InChI:InChI=1/C4H6O/c1-2-3-4-5/h5H,4H2,1H3
764-01-2 Relevant articles
BiCl3-Facilitated removal of methoxymethyl-ether/ester derivatives and DFT study of -O-C-O- bond cleavage
Pacherille, Angela,Tuga, Beza,Hallooman, Dhanashree,Dos Reis, Isaac,Vermette, Mélodie,Issack, Bilkiss B.,Rhyman, Lydia,Ramasami, Ponnadurai,Sunasee, Rajesh
supporting information, p. 7109 - 7116 (2021/05/03)
A simple method for the cleavage of meth...
Synthesis and Photoswitching Properties of Bioinspired Dissymmetric I-Pyrone, an Analogue of Cyclocurcumin
Pecourneau, Jérémy,Losantos, Raúl,Monari, Antonio,Parant, Stéphane,Pasc, Andreea,Mourer, Maxime
, p. 8112 - 8126 (2021/06/30)
Cyclocurcumin (CC), a turmeric curcumino...
Catalytic asymmetric synthesis of 2,5-dihydrofurans using synergistic bifunctional Ag catalysis
Shi, Taoda,Teng, Shenghan,Gopi Krishna Reddy, Alavala,Guo, Xin,Zhang, Yueteng,Moore, Kohlson T.,Buckley, Thomas,Mason, Damian J.,Wang, Wei,Chapman, Eli,Hu, Wenhao
supporting information, p. 8737 - 8744 (2019/10/16)
We report a bifunctional Ag catalyst pro...
Nickel(II)-Catalyzed Asymmetric Propargyl [2,3] Wittig Rearrangement of Oxindole Derivatives: A Chiral Amplification Effect
Xu, Xi,Zhang, Jianlin,Dong, Shunxi,Lin, Lili,Lin, Xiaobin,Liu, Xiaohua,Feng, Xiaoming
supporting information, p. 8734 - 8738 (2018/07/14)
A highly enantioselective [2,3] Wittig r...
764-01-2 Process route
-

-
37428-46-9,37428-53-8,40605-42-3
3-chloro-2-buten-1-ol

-
-
764-01-2
methyl propargyl alcohol
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
In
cyclohexane; water;
at 65 - 72 ℃;
for 8h;
|
88.1% |
|
With
potassium hydroxide;
tetra-(n-butyl)ammonium iodide;
In
hexane;
for 12h;
Heating;
|
50% |
|
With
ammonia; sodium; ferric nitrate;
for 14h;
|
47% |
|
With
ammonia; sodium amide;
Behandeln des Reaktions-Loesung mit NH4Cl;
|
|
|
With
ammonia; sodium amide;
|
-
-
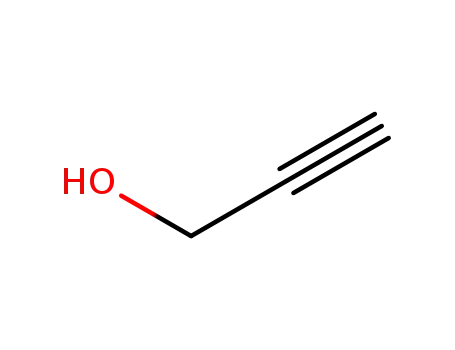
107-19-7
propargyl alcohol

-
-
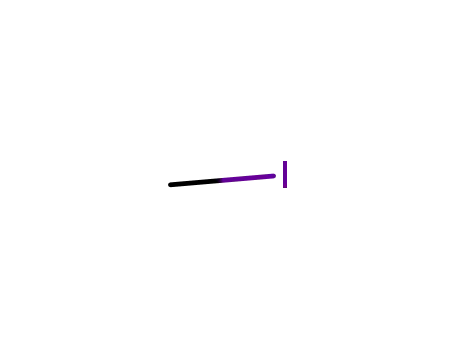
74-88-4
methyl iodide

-

-
764-01-2
methyl propargyl alcohol
| Conditions | Yield |
|---|---|
|
With
lithium; ferric nitrate;
In
ammonia;
at -50 ℃;
for 2h;
|
95% |
|
With
lithium amide;
In
ammonia;
|
78% |
|
Multistep reaction;
|
|
|
methyl iodide;
With
bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine;
at 25 ℃;
for 0.166667h;
Inert atmosphere;
propargyl alcohol;
at 25 ℃;
for 10h;
Inert atmosphere;
|
764-01-2 Upstream products
-
50-00-0
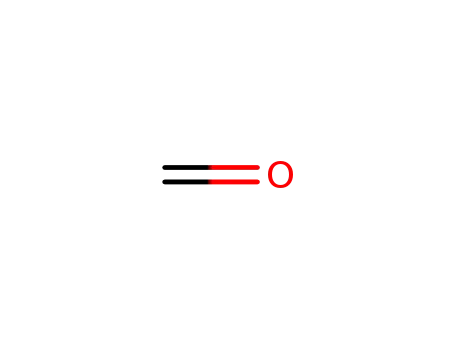
formaldehyd
-
16466-97-0
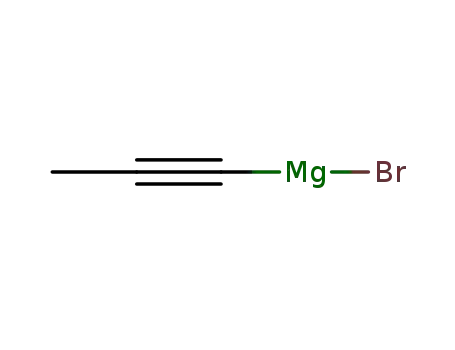
1-propynylmagnesium bromide
-
37428-46-9
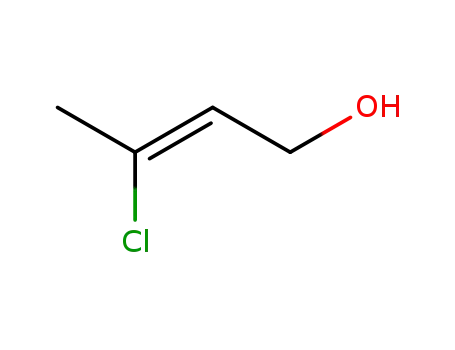
(Z)-3-chloro-2-but-en-1-ol
-
4089-67-2
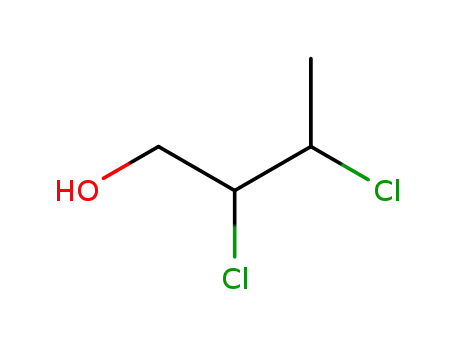
2,3-dichloro-1-butanol
764-01-2 Downstream products
-
504-61-0
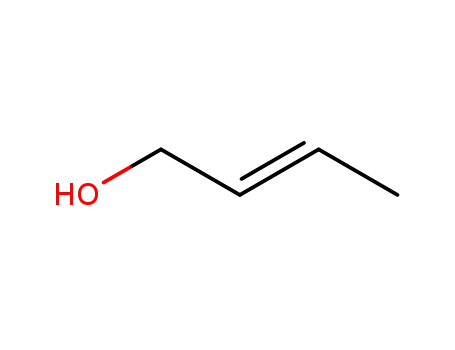
(E)-but-2-enol
-
4088-60-2
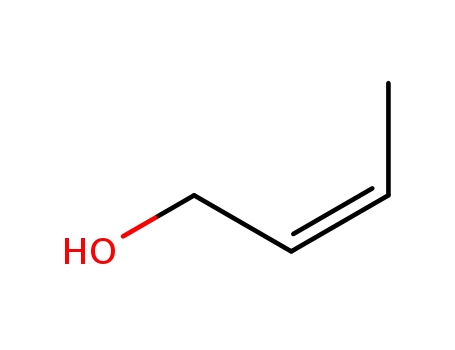
cis-2-buten-1-ol
-
590-93-2
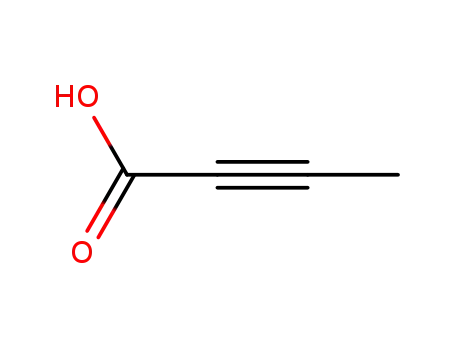
2-Butynoic acid
-
3355-17-7
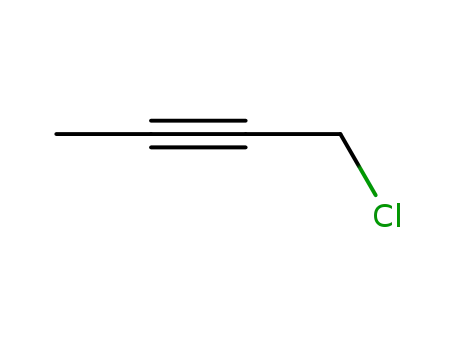
1-chloro-2-butyne


