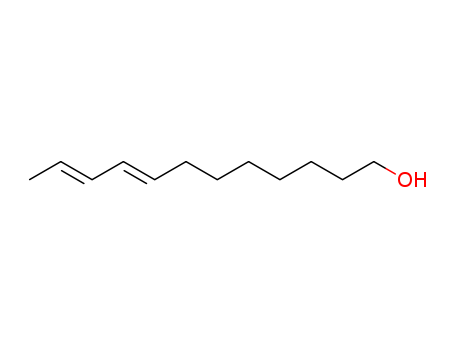
8,10-DODECADIEN-1-OL
-
Product Name :
8,10-DODECADIEN-1-OL
-
CAS No :
33956-49-9
-
Project State :
Commercial
Application
General Description
Cost-effective customized wholesale 8,10-DODECADIEN-1-OL 33956-49-9
- Molecular Formula:C12H22 O
- Molecular Weight:182.306
- Vapor Pressure:0.000886mmHg at 25°C
- Melting Point:30-32 °C
- Refractive Index:1.473
- Boiling Point:270.7°Cat760mmHg
- PKA:15.19±0.10(Predicted)
- Flash Point:100.9°C
- PSA:20.23000
- Density:0.862g/cm3
- LogP:3.45160
8,10-DODECADIEN-1-OL(Cas 33956-49-9) Usage
|
Synthesis |
To a mixed so lution of Methyl5-oxo-(E,E)-8,10-dodecadienoate (15.7g, 0.07 mol) and p-toluene sulfonylhydrazine (16.3g, 0.0875 mol) in DMF - sulfolane (350ml, 1 : 1) was added p-toluenesulfonic acid (1.75g) and sodium cyanoborohydride (17.6g, 0.28mol) at room temperature, and the solution was heated at 100 ℃ for 4 hr. After cooling to room temperature, the reaction mixture was diluted with water (1000ml) and extracted with cyclohexane. The cyclohexane solution was washed with water, dried over Na2SO4 and concentrated in vacuo. Column chromatography of the oily residue over silica gel (Merck Kieselgel 60) with petroleum ether-ether (10:1) gave 4 (5.6g, 38%) as a pale yellow liquid.A solution of Methyl (E,E)-8,10-dodecadienoate (4.2g, 0.02mol) in absolute ether (40ml) was added dropwise to a slurry of lithium aluminum hydride (0.38g, 0.01mol) in absolute ether (60 ml) with stirring at room temperature. Stirring was continued for 1 hr at room temperature. After the mixture was refluxed for I hr, the reaction was quen ched with 2NH2SO4 (30ml) and the mixture was worked up by the usual procedure to give the alcohol 5, with a nearly quantitative yield, which solidified during storage in a refrigerator, (recrystallized from pe troleum ether). The purity was 97% on GLC analysis. |
InChI:InChI=1/C12H22O/c1-2-3-4-5-6-7-8-9-10-11-12-13/h2-5,13H,6-12H2,1H3/b3-2+,5-4+
33956-49-9 Relevant articles
Insect pheromones and their analogs I. Synthesis of the sex attractant of the codling moth
Tolstikov,Dzhemilev,Khusnutdinov
, p. 101 - 102 (1978)
The synthesis of the sex attractant of t...
A GENERAL AND STEREOSELECTIVE SYNTHESIS OF (E,E)-CONJUGATED DIENES.
Bloch, R.,Abecassis, J.
, p. 1247 - 1250 (1983)
Cis-2,5-disubstituted-2,5-dihydrothiophe...
Synthesis of dodeca-8E,10E-dien-1-ol - The sex pheromone of Laspeyresia pomonella via the acetolysis of 4-propenyl-1,3-dioxane
Shakova,Zorin,Musavirov,Safarov,Muslukhov,Kharisov,Ishmuratov,Rakhmankulov
, p. 582 - 584 (1996)
A new scheme has been developed for the ...
A new synthesis of (E,E)-8,10-dodecadien-1-ol, sex pheromone of coding moth
Naoshima,Nakagawa,Wakabayashi,Hayashi
, p. 1419 - 1420 (1980)
-
Stereoselective Cross-Coupling of Grignard Reagents and Conjugated Dienylbromides using Iron Salts with Magnesium Alkoxides
Chourreu, Pablo,Gayon, Eric,Guerret, Olivier,Guillonneau, Lo?c,Lefèvre, Guillaume
supporting information, p. 4701 - 4706 (2021/09/10)
A convenient procedure allowing iron-cat...
Synthesis method of laspeyresia pomonella sex pheromone
-
Paragraph 0030; 0037; 0038; 0039; 0046; 0047, (2019/09/17)
The invention relates to a synthesis met...
SYNTHESIS OF PHEROMONES AND RELATED MATERIALS VIA OLEFIN METATHESIS
-
, (2018/09/12)
Methods for preparation of olefins, incl...
(8E, 10E) - 8,10-dodecadienol-1-ol for the preparation of
-
Paragraph 0024; 0025, (2017/08/04)
The invention discloses a preparation me...
33956-49-9 Process route
-
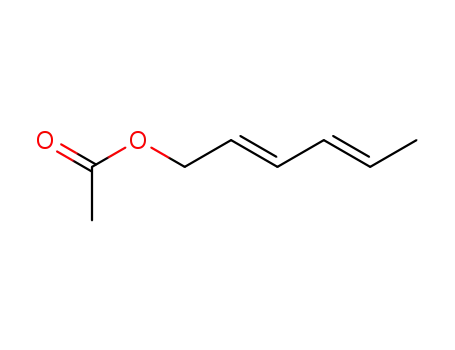
-
57006-69-6
(E,E)-sorbyl acetate

-
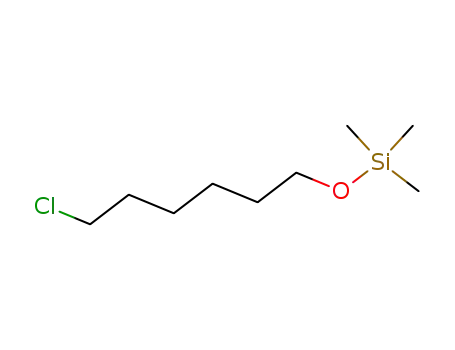
-
34714-00-6
trimethylsilyl 6-chloro-1-hexyl ether

-
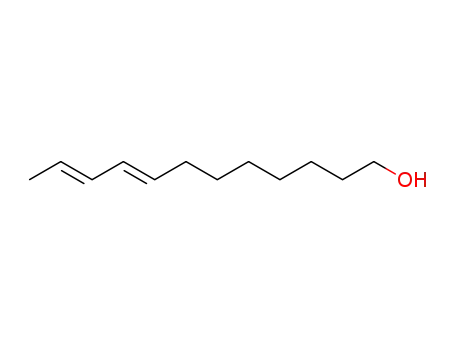
-
33956-49-9
(8E,10E)-dodeca-8,10-dienol
| Conditions | Yield |
|---|---|
|
(E,E)-sorbyl acetate; trimethylsilyl 6-chloro-1-hexyl ether;
With
sodium;
In
toluene;
at 0 - 80 ℃;
for 12h;
Inert atmosphere;
Green chemistry;
With
sulfuric acid;
In
water; toluene;
at 0 - 20 ℃;
for 1h;
Temperature;
Green chemistry;
|
77.47% |
-
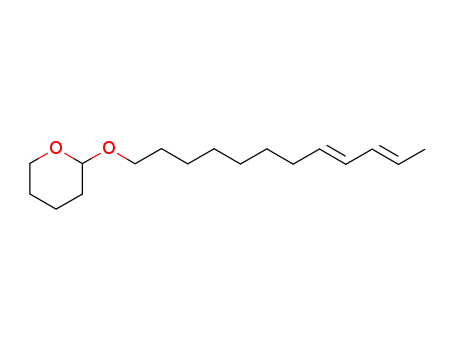
-
37935-49-2
(8E,10E)-8,10-Dodecadienyl-(tetrahydro-2-pyranyl)ether

-

-
33956-49-9
(8E,10E)-dodeca-8,10-dienol
| Conditions | Yield |
|---|---|
|
With
toluene-4-sulfonic acid;
In
methanol; water;
at 60 ℃;
for 2h;
Yield given;
|
|
|
With
toluene-4-sulfonic acid;
In
methanol;
at 50 ℃;
for 2h;
Yield given;
|
|
|
With
methanesulfonic acid; water;
In
methanol;
at 60 ℃;
for 3h;
|
0.74 g |
|
With
toluene-4-sulfonic acid;
In
methanol;
at 70 ℃;
for 0.5h;
|
33956-49-9 Upstream products
-
629-11-8
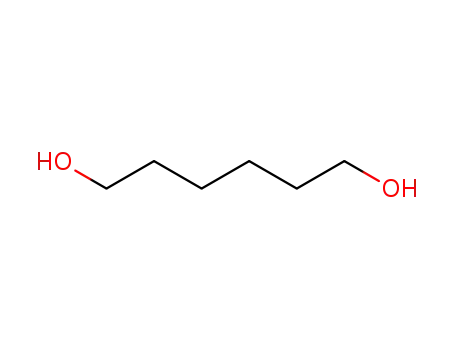
1,6-hexanediol
-
57006-69-6

(E,E)-sorbyl acetate
-
123-73-9
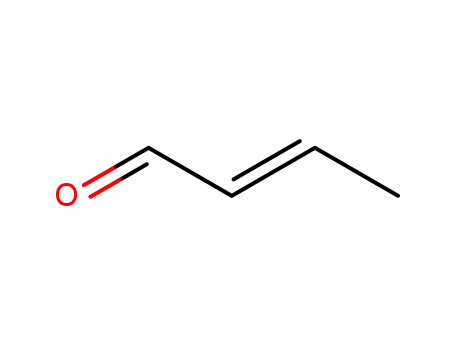
trans-Crotonaldehyde
-
65734-62-5
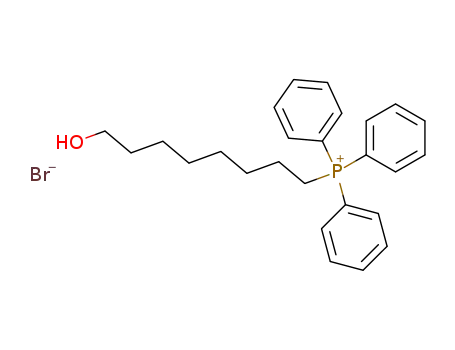
8-hydroxy-octyl-triphenyl-phosphonium bromide
33956-49-9 Downstream products
-
67992-59-0
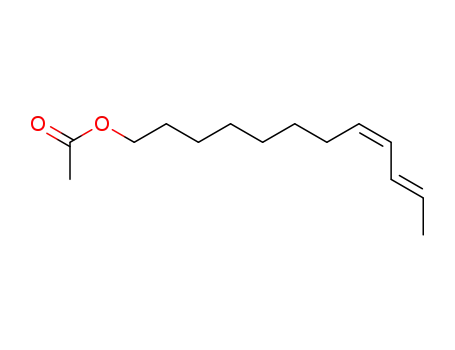
(8Z,10E)-8,10-dodecadienyl acetate
-
67992-60-3
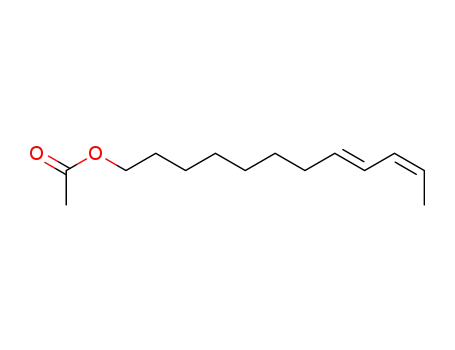
(8E,10Z)-dodeca-8,10-dien-1-yl acetate
-
53880-51-6
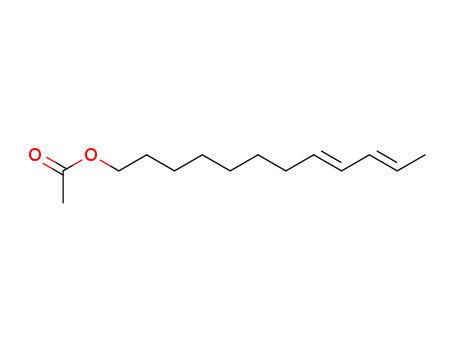
(E,E)-8,10-dodecadienyl acetate
-
86217-90-5
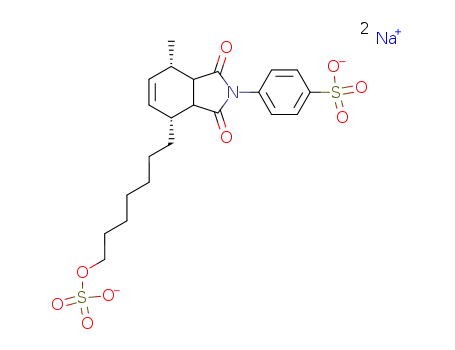
3a,4,7,7a-Tetrahydro-2-(4-sodium sulfophenyl)-4β-(7-sodium sulfatoheptyl)-7β-methyl-1H-isoindole-1,3(2H)-dione


