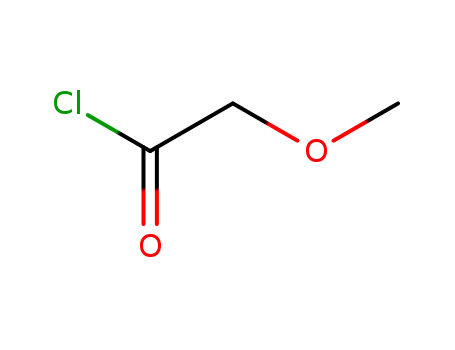
Methoxyacetyl chloride
-
Product Name :
Methoxyacetyl chloride
-
CAS No :
38870-89-2
-
Project State :
Commercial
Application
General Description
Bulk supply high purity Methoxyacetyl chloride 38870-89-2, Paid sample available
- Molecular Formula:C3H5ClO2
- Molecular Weight:108.525
- Appearance/Colour:clear colorless to yellow liquid
- Vapor Pressure:21.7mmHg at 25°C
- Melting Point:<-40 °C
- Refractive Index:n20/D 1.419(lit.)
- Boiling Point:112.5 °C at 760 mmHg
- Flash Point:28.9 °C
- PSA:26.30000
- Density:1.167 g/cm3
- LogP:0.39820
Methoxyacetyl chloride(Cas 38870-89-2) Usage
InChI:InChI=1/C3H5ClO2/c1-6-2-3(4)5/h2H2,1H3
38870-89-2 Relevant articles
A Diels-Alder reaction for the total synthesis of the novel antibiotic antitumor agent mensacarcin
Tietze, Lutz F.,Guentner, Carlos,Gericke, Kersten M.,Schuberth, Ingrid,Bunkoczi, Gabor
, p. 2459 - 2467 (2005)
The antibiotic mensacarcin (1), which co...
Chain hydrocarbon substituted isoindoline -1, 3 - diketone PDE4 inhibitor and medicinal use thereof
-
Paragraph 0259-0262, (2021/10/27)
The invention relates to a compound show...
Preparation method and application of iopromide intermediate (by machine translation)
-
Paragraph 0056-0058, (2020/07/24)
The invention relates to a preparation m...
Synthesis and application of peptide borate compounds
-
Paragraph 0163; 0179-0181, (2019/12/25)
The invention belongs to the field of dr...
Synthesis method of optically active metalaxyl
-
Paragraph 0031-0035; 0041-0045; 0051-0055; 0061-0065; 0071, (2019/01/21)
The invention discloses a synthesis meth...
38870-89-2 Process route
-
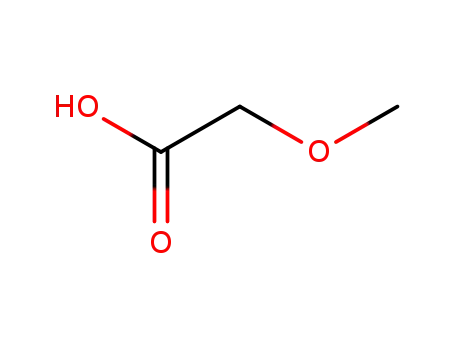
-
625-45-6
2-methoxyacetic acid

-
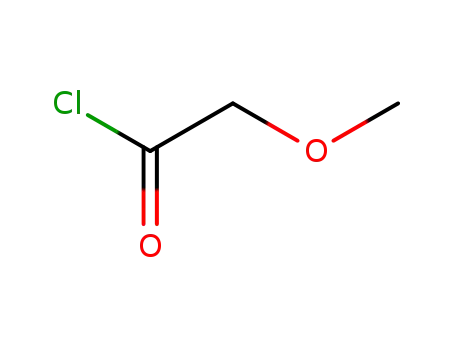
-
38870-89-2
Methoxyacetyl chloride
| Conditions | Yield |
|---|---|
|
With
oxalyl dichloride;
N,N-dimethyl-formamide;
In
dichloromethane;
at 0 - 20 ℃;
|
90% |
|
With
oxalyl dichloride; N,N-dimethyl-formamide;
In
dichloromethane;
at 0 - 20 ℃;
|
90% |
|
With
pyridine; thionyl chloride;
at 80 ℃;
|
86% |
|
With
thionyl chloride;
In
N,N-dimethyl-formamide;
for 2.5h;
Heating;
|
83% |
|
With
thionyl chloride;
In
dichloromethane;
at 0 - 20 ℃;
for 2h;
|
82% |
|
With
thionyl chloride;
for 2h;
Heating;
|
81% |
|
With
thionyl chloride;
for 1h;
Heating;
|
69% |
|
With
thionyl chloride;
at 110 - 120 ℃;
for 2h;
|
63% |
|
With
thionyl chloride;
at 50 - 60 ℃;
|
|
|
With
hydrogenchloride; thionyl chloride;
|
|
|
With
thionyl chloride;
|
|
|
With
thionyl chloride;
|
|
|
With
thionyl chloride;
at 50 - 60 ℃;
for 0.5h;
|
6.10 g |
|
With
1,1,1,3,3,3-hexachloro-propan-2-one; triethyl phosphite;
In
dichloromethane;
at 0 ℃;
for 0.5h;
|
|
|
With
oxalyl dichloride;
In
dichloromethane; N,N-dimethyl-formamide;
at 20 ℃;
for 1h;
|
|
|
With
oxalyl dichloride;
In
dichloromethane; N,N-dimethyl-formamide;
at 20 ℃;
for 1.5h;
|
|
|
With
anhydrous phosphorus trichloride;
|
|
|
With
oxalyl dichloride; N,N-dimethyl-formamide;
In
dichloromethane;
at 20 ℃;
|
|
|
With
thionyl chloride;
In
chloroform;
at 0 - 40 ℃;
|
|
|
With
thionyl chloride;
|
|
|
With
thionyl chloride; N,N-dimethyl-formamide;
In
dichloromethane;
at 0 - 80 ℃;
|
|
|
With
thionyl chloride;
In
dichloromethane;
at 80 ℃;
|
|
|
With
oxalyl dichloride;
In
dichloromethane;
Reflux;
|
|
|
With
oxalyl dichloride; N,N-dimethyl-formamide;
In
dichloromethane;
at 0 - 20 ℃;
Inert atmosphere;
|
|
|
With
oxalyl dichloride; N,N-dimethyl-formamide;
In
dichloromethane;
at 0 - 20 ℃;
Inert atmosphere;
|
10.9 g |
|
With
thionyl chloride; N,N-dimethyl-formamide;
In
dichloromethane;
at 20 ℃;
for 4h;
Inert atmosphere;
|
|
|
With
thionyl chloride;
In
1,2-dichloro-ethane; N,N-dimethyl-formamide;
at 0 - 80 ℃;
for 2h;
Inert atmosphere;
|
|
|
With
thionyl chloride;
In
1,4-dioxane;
at 0 - 10 ℃;
for 1h;
|
|
|
With
1-chloro-1-(dimethylamino)-2-methyl-1-propene;
In
chloroform-d1;
at 20 ℃;
Inert atmosphere;
|
100 %Spectr. |
|
With
oxalyl dichloride; N,N-dimethyl-formamide;
In
dichloromethane;
Inert atmosphere;
|
|
|
With
thionyl chloride;
at 25 - 30 ℃;
for 3.5h;
Inert atmosphere;
Green chemistry;
|
|
|
With
thionyl chloride;
In
dichloromethane; N,N-dimethyl-formamide;
at 10 - 20 ℃;
for 12h;
|
|
|
With
oxalyl dichloride;
In
dichloromethane; N,N-dimethyl-formamide;
at 20 ℃;
|
|
|
With
thionyl chloride;
for 1h;
Heating / reflux;
|
|
|
With
thionyl chloride;
In
dichloromethane;
at -10 - 20 ℃;
for 2h;
|
|
|
With
thionyl chloride; N,N-dimethyl-formamide;
In
dichloromethane;
at 10 - 20 ℃;
for 12h;
|
|
|
With
phosgene;
In
dichloromethane; N,N-dimethyl-formamide;
at 0 - 20 ℃;
for 18h;
|
-
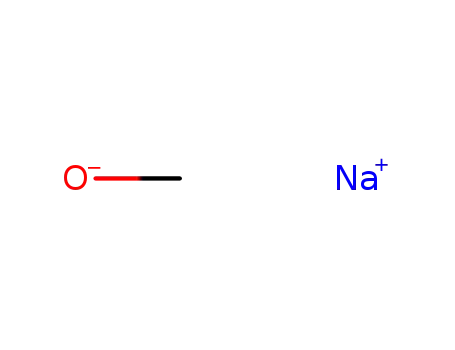
-
124-41-4
sodium methylate

-
-
79-11-8
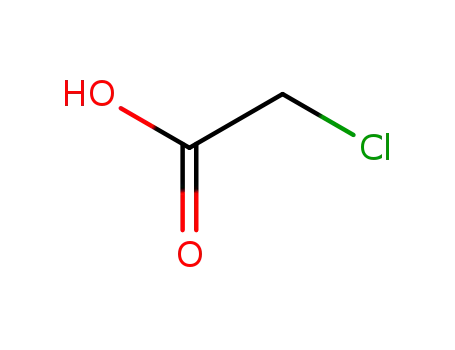
chloroacetic acid

-

-
38870-89-2
Methoxyacetyl chloride
| Conditions | Yield |
|---|---|
|
sodium methylate; chloroacetic acid;
In
methanol;
at 58 ℃;
for 0.5h;
With
phosphorus trichloride;
In
toluene;
at 35 - 40 ℃;
for 1.78333h;
Temperature;
|
38870-89-2 Upstream products
-
98-88-4
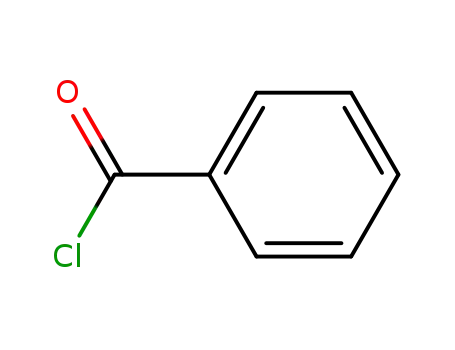
benzoyl chloride
-
625-45-6

2-methoxyacetic acid
-
201230-82-2
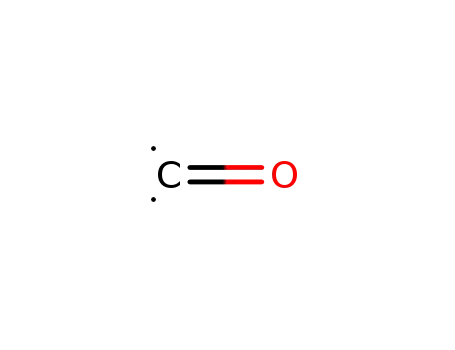
carbon monoxide
-
107-30-2
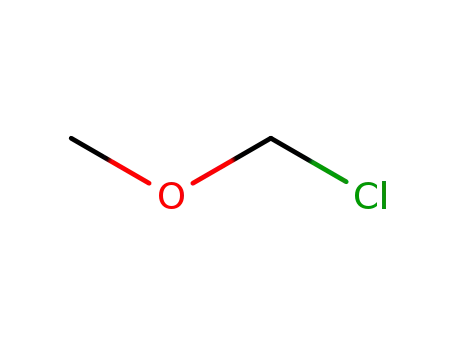
chloromethyl methyl ether
38870-89-2 Downstream products
-
32786-24-6
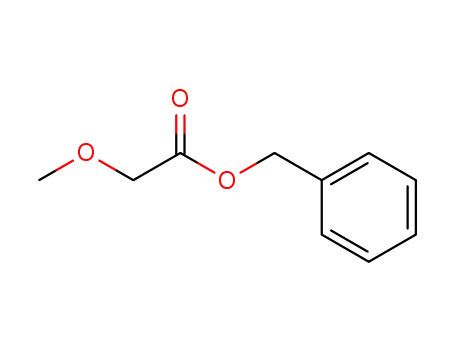
benzyl 2-methoxyacetate
-
84682-19-9
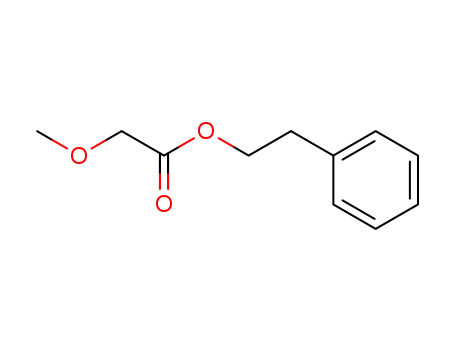
2-methoxyacetic acid 2-phenylethyl ester
-
109595-32-6
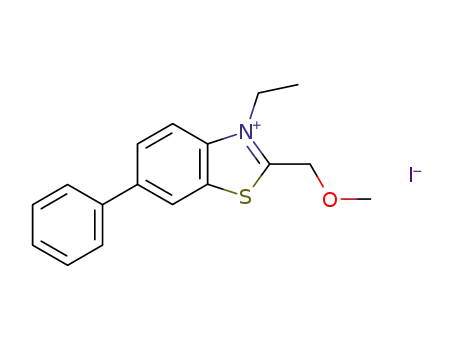
3-ethyl-2-methoxymethyl-6-phenyl-benzothiazolium; iodide
-
103209-94-5
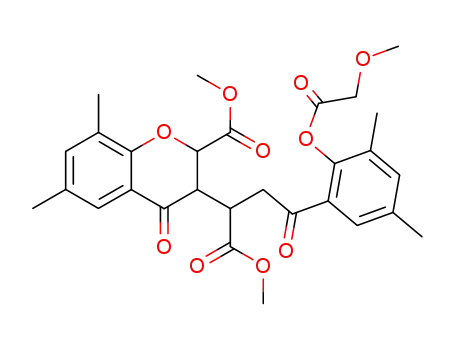
4-(2-methoxyacetoxy-3,5-dimethyl-phenyl)-2-(2-methoxycarbonyl-6,8-dimethyl-4-oxo-chroman-3-yl)-4-oxo-butyric acid methyl ester


