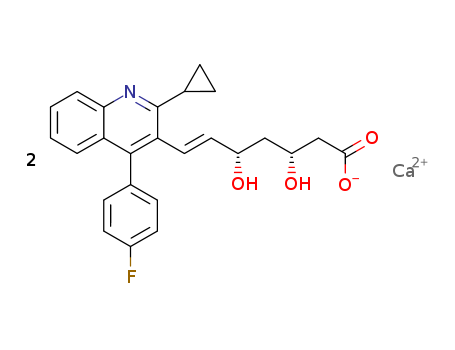
Pitavastatin calcium
-
Product Name :
Pitavastatin calcium
-
CAS No :
147526-32-7
-
Project State :
Commercial
Application
General Description
Buy cost-effective 99% pure Pitavastatin calcium 147526-32-7 now
- Molecular Formula:(C25H23FNO4)2.Ca
- Molecular Weight:880.999
- Appearance/Colour:white to off-white powder
- Boiling Point:692 °C at 760 mmHg
- Flash Point:372.3 °C
- PSA:186.96000
- LogP:6.36680
Pitavastatin calcium(Cas 147526-32-7) Usage
|
Statin lipid-lowering drugs |
Pitavastatin calcium is jointly developed by two companies Nissan Chemical and Kowa Co.it is the first total synthesis HMG-CoA reductase inhibitor, it belongs to statin drugs ,it reduces the ability of the liver to manufacture cholesterol mainly through inhibition of some liver enzymes called HMGCo-A reductase , thus it improves the elevated blood cholesterol levels, it is primarily used for the treatment of hypercholesterolemia and familial hypercholesterolemia patients,its lipid-lowering effect is very good, it is the most potent lipid-lowering drug so far. |
|
Pharmacological effects |
Inhibition of HMG-CoA reductase: pitavastatin calcium has a strongly antagonistic inhibition effect on HMG-CoA enzyme , IC50 value is 6.8 nmol/L, and its intensity is 24 times of simvastatin, while it is 68 times of fluvastatin. It Hinders the synthesis of cholesterol: the ability to effectively inhibit the process of generating cholesterol in human hepatocytes HepG2 , IC50 value is 5.8nmol/L, and its intensity is 29 times of simvastatin, it is 57 times that of atorvastatin. But pitavastatin calcium inhibition effect on each enzyme in cholesterol generation after generation of mevalonate is very weak. It Increases LDL receptor density: pitavastatin induces the synthesis of LDS receptor mRNA in the ultra-low concentration of 1μmol/L, it can increase the number of LDS receptor mRNA , it results in the increasing of LDL receptor density , thereby it promotes clearance of LDL , so that plasma LDL-plasma total cholesterol concentration and triglyceride concentration decrease. The above information is edited by the lookchem of Tian Ye. |
|
Pharmacokinetics |
The main parts of its absorption after oral administration are the duodenum and colon,its rate of binding plasma protein in the body is 96%and it is more selectively distributed in the liver after absorption , the drug concentration in body tissues is lower than that in the plasma or the same as that in the plasma . Pitavastatin calcium is mainly metabolized in the liver, kidney, lung, heart, muscle , metabolite concentrations are lower than the concentration of drug prototype, it is excreted through feces,there is also a small amount of drug excretion through urine , total excretion rate is almost 100%. A healthy male adult oral pitavastatin is 0.5~8mg, t1/2 is about 10h,the cmax and AUC of the prototype drug in plasma increase with increasing dose , repeatedly taking does not result in medication savings. |
|
Toxicity |
Acute toxicity: rats and dogs oral,study its acute toxicity. Pitavastatin calcium median lethal dose on the rats are about male 500~1000mg/kg, female 250~500 mg/kg, dogs lethal dose is about 50~100mg/kg. Long term toxicity: respectively, rats, dogs and monkeys are administered a long-term experiment. From the experimental results, the safe dose of pitavastatin calcium are rats 1 mg/kg · d-1 (6 months), canine 0.3mg/kg · d-1 (12 months), monkey 3mg/kg · d-1 (6 months). No central nervous system, reproductive system, and myocardial dysfunction is observed which is common while taking other statins during administration. Carcinogenicity, mutagenicity: mouse oral 1,12,30, 75mg/kg dose, the incidence of cancer has no significant increase than in the control group .in Chromosome abnormality tests, at the highest concentration of 625μg/ml,the result is positive, but at the same concentration, gene mutation recovery tests, micronucleus test s(in vivo) and UDS test s(in vivo) are negative. |
|
Clinical Study |
In the therapeutic effect, statins is the first In the lipid-lowering drugs in which pitavastatin effect is very obvious, pitavastatin calcium is a third generation statins anti-hyperlipidemia drug, and Russell atorvastatin (rosuvastatin ) while being called "super statin", is one of the better statins which are current international clinical application of the treatment of hypercholesterolemia, familial hypercholesterolemia , because its clinically effective dose is 1-2mg/day, significantly lower than other marketed statins, with high efficiency, and security features, it has a good tolerability. clinical trail phase I results show that pitavastatin calcium in 1,2,4 mg dose has clinical significance in patients with high blood cholesterol. Results of clinical trail phase Ⅱ determine that the best dosage of the pitavastatin calcium for the treatment of hyperlipidemia is 2mg/d. clinical trail phaseⅢ comparison of experimental results show that the efficacy of pitavastatin calcium on reducing Tc and LDL-c is better than the effect of fluvastatin, and there is no significant difference in safety. Multi-center long-term administration tests carried out in Japan show that the dosage in 1~4mg/d range can effectively control blood lipid levels. The above test results demonstrate the effectiveness of pitavastatin calcium in treatment of hyperlipidemia on clinic. |
|
Hazard |
A poison by ingestion. |
|
Safety Profile |
A poison by ingestion.Experimental reproductive effects. When heated todecomposition it emits toxic vapors of NOx and Fí. |
|
Synthesis |
The convergent synthesis was achieved by cross-coupling of aryl halide 149 with (E)- alkenyl borane 155 which was derived from terminal acetylene 154 by via hydroboration. Anthranilic acid (143) was treated with TsCl and sodium carbonate in hot water to give N-tosylated intermediate in 78% yield, which was converted to the corresponding acid chloride 144 with PCl5 in o-dichlorobenzene at 85°C. Intermediate 144, without isolation, was reacted with fluorobenzene in the presence of AlCl3 at 80°C to give the Friedel-Crafts product which was then hydrolyzed in hot water to give fluorobenzophenone free aniline 145 in 64% yield from the N-tosyl anthranilic acid. Acetyl cyclopropane (146) was reacted with diethyl carbonate to give the corresponding ethyl ester 147. The quinoline core structure was obtained by condensing fluorobenzophenone 145 with 147 under acidic conditions with a Dean-Stark trap to give quinoline-3- carboxylic ethyl ester 148 in 90% yield. Ester 148 was hydrolyzed with potassium hydroxide, and the free carboxylic acid thus obtained was subsequently photoiododecarboxylated with iodine and PhI(OAc)2 to give aryl iodide 149 in 74% yield. 3-Trimethylsilylpropynal (150) was used as the starting material to prepare the chiral side chain. Compound 150 was reacted with di-anion 151 in THF at low temperature to give the corresponding diol ester which was first reacted with Et2BOMe and then reduced to acetylene with sodium borohydride. The free diol was protected as ketal with 2,2-dimethoxypropane in the presence of TsOH to give dimethylketal acetylene 152 in 99% yield. The ester functionality was hydrolyzed with sodium hydroxide to give the acid in 92% yield. The racemic free acid was resolved with (R)-(1-naphthyl)ethylamine to give the pure diastereomeric salt 153 which crystallized out in 31% yield and 97% e.e. Esterification of the free carboxylic acid liberated from the crystalline salt with ethyl iodide gave optically pure acetylene 154 in 70% yield. Hydroboration of acetylene 154 with disiamylborane gave (E)-alkenyldisiamylborane 155 and the excess borane reagent was quenched with sodium ethoxide in ethanol. After evaporation of all volatile material, the residue was directly subjected to the cross-coupling reaction. Palladium (II) chloride and aryl iodide 149 were mixed in acetonitrile to give coupling product 156 in 99% yield. After the ketal in 156 was hydrolyzed under acid conditions and the ester was hydrolyzed with sodium hydroxide, the resulting carboxylic sodium salt was reacted with calcium chloride to yield pitavastatin calcium (XIX) with 99% e.e. |
|
Definition |
ChEBI: The calcium salt of pitavastatin. Used for treatment of hypercholesterolemia (elevated levels of cholesterol in the blood) on patients unable to sufficiently lower their cholesterol levels by diet and exercise. |
|
Brand name |
Livalo |
InChI:InChI=1/2C25H25FNO4.Ca/c2*26-17-9-7-15(8-10-17)24-20-3-1-2-4-22(20)27-25(16-5-6-16)21(24)12-11-18(28)13-19(29)14-23(30)31;/h2*1-4,7-12,16,18-19,23,28-30H,5-6,13-14H2;/q2*-1;+2/b2*12-11+;/t2*18-,19-,23+;/m11./s1
147526-32-7 Relevant articles
Method for preparing rosuvastatin and pitavastatin 2, 5-diene heptanoate compound
-
Paragraph 0031-0033, (2020/05/14)
The invention discloses a method for pre...
Multi-substituted dihydroisoquinolines he the sandbank contains the fluorine derivative and use thereof
-
Paragraph 0135; 0136, (2018/06/04)
The invention belongs to the field of ph...
Palladium-Catalyzed Stereoselective Cyclization of in Situ Formed Allenyl Hemiacetals: Synthesis of Rosuvastatin and Pitavastatin
Spreider, Pierre A.,Breit, Bernhard
, p. 3286 - 3290 (2018/06/11)
A diastereoselective palladium-catalyzed...
New crystal form of pitavastatin hemicalcium salt and preparation method thereof
-
Paragraph 0052-0061, (2019/01/08)
The invention provides a new crystal for...
147526-32-7 Process route
-
-
147526-32-7
pitavastatin calcium salt

-
-
147526-32-7,147511-69-1,688735-41-3,769908-13-6,121659-03-8
pitavastatin
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
water; ethyl acetate;
at 25 - 35 ℃;
|
95.9% |
|
With
hydrogenchloride;
In
dichloromethane; water;
at 0 - 25 ℃;
for 0.833333h;
pH=3;
|
|
|
With
hydrogenchloride;
In
water; ethyl acetate;
pH=4;
|
3.7 g |
|
With
hydrogenchloride;
In
dichloromethane; water;
|
-
![(4R,6S)-(E)-{6-[2-(2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)-vinyl]-2,2-dimethyl-1,3-dioxan-4-yl}acetic acid tert-butyl ester](/upload/2025/9/f6b181bf-f23b-4f8e-851e-49e48685eb17.png)
-
147489-06-3
(4R,6S)-(E)-{6-[2-(2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)-vinyl]-2,2-dimethyl-1,3-dioxan-4-yl}acetic acid tert-butyl ester

-

-
147526-32-7,147511-69-1,688735-41-3,769908-13-6,121659-03-8
pitavastatin
| Conditions | Yield |
|---|---|
|
(4R,6S)-(E)-{6-[2-(2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)-vinyl]-2,2-dimethyl-1,3-dioxan-4-yl}acetic acid tert-butyl ester;
With
trifluoroacetic acid;
In
acetonitrile;
at 30 - 35 ℃;
With
caesium carbonate;
In
acetonitrile;
at 35 - 40 ℃;
|
94% |
|
Multi-step reaction with 3 steps
1.1: water; oxalic acid / methanol / 6 h / 35 °C
1.2: 2.75 h / 10 - 30 °C / pH 7
2.1: sodium hydroxide; water / methanol / 1.67 h / 0 °C
2.2: 0.67 h / 25 °C
3.1: hydrogenchloride / dichloromethane; water / 0.83 h / 0 - 25 °C / pH 3
With
hydrogenchloride; water; oxalic acid; sodium hydroxide;
In
methanol; dichloromethane; water;
|
|
|
Multi-step reaction with 4 steps
1.1: water; oxalic acid / methanol / 6 h / 35 °C
1.2: 2.75 h / 10 - 30 °C / pH 7
2.1: sodium hydroxide; water / acetonitrile / 1.5 h / 30 °C
2.2: 0.25 h / 0 °C / pH 4
2.3: 0.5 h / 0 °C
3.1: sodium hydroxide / water / 0.75 h / 30 °C
3.2: 0.75 h / 35 °C
4.1: hydrogenchloride / dichloromethane; water / 0.83 h / 0 - 25 °C / pH 3
With
hydrogenchloride; water; oxalic acid; sodium hydroxide;
In
methanol; dichloromethane; water; acetonitrile;
|
|
|
Multi-step reaction with 2 steps
1: hydrogenchloride / acetonitrile; water / 2 h / 13 °C
2: sodium hydroxide / water / 2 h / 10 °C / Large scale
With
hydrogenchloride; sodium hydroxide;
In
water; acetonitrile;
|
147526-32-7 Upstream products
-
172336-32-2
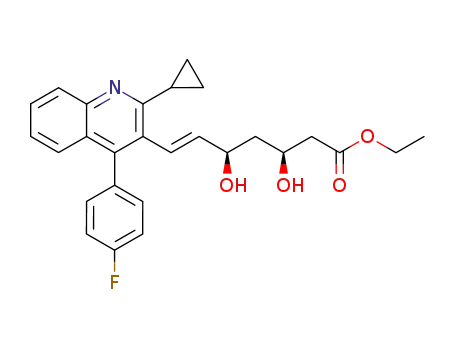
(±)-E-3,5-dihydroxy-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl]-6-heptenoic acid ethyl ester
-
121660-11-5
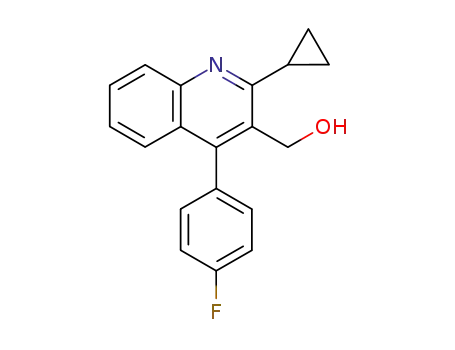
2-cyclopropyl-4-(4-fluorophenyl)-3-(hydroxymethyl)quinoline
-
121660-37-5
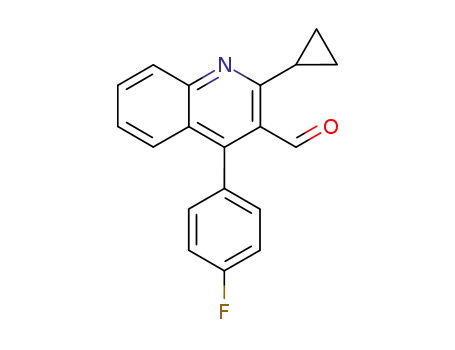
2-cyclopropyl-4-(4-fluorophenyl)-3-formylquinoline
-
148901-68-2
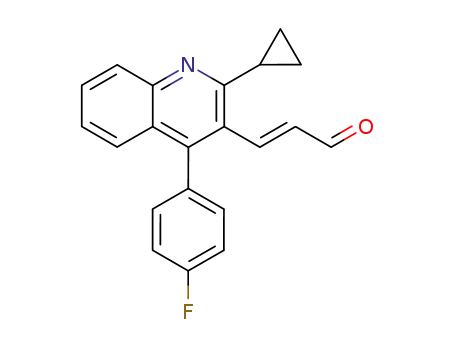
(E)-3-[2-cyclopropyl-4-(4-fluoro-phenyl)-quinolin-3-yl]-propenal
147526-32-7 Downstream products
-
1213706-08-1
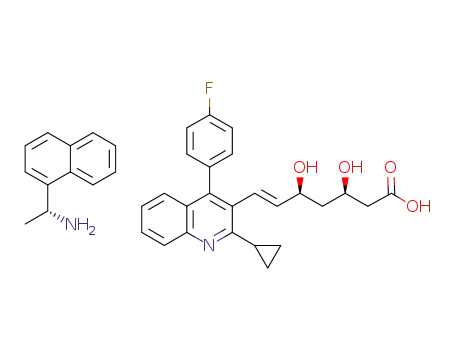
(3R,5S,E)-7-(2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)-3,5-dihydroxyhept-6-enoic acid (R)-NEA salt
-
1392469-72-5
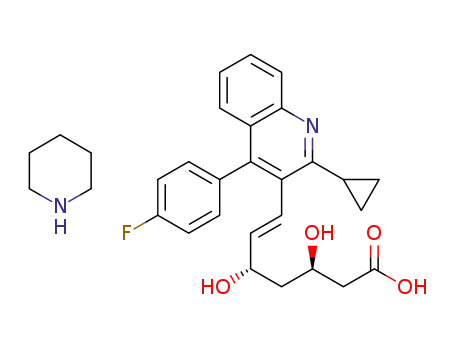
pitavastatin piperidine
-
1392469-76-9
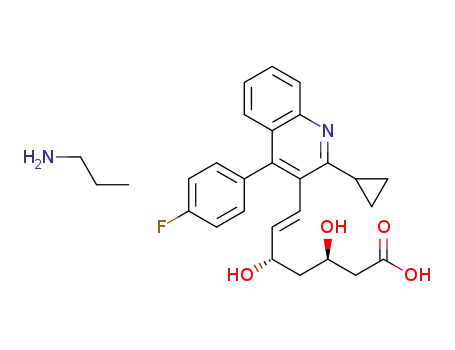
pitavastatin n-propylamine
-
1353637-19-0

(3R,5S,6E)-7-[2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl]-3,5-dihydroxyhept-6-enoic acid diisopropylamine salt


