tert-Butyl (4R-cis)-6-formaldehydel-2,2-dimethyl-1,3-dioxane-4-acetate
-
Product Name :
tert-Butyl (4R-cis)-6-formaldehydel-2,2-dimethyl-1,3-dioxane-4-acetate
-
CAS No :
124752-23-4
-
Project State :
Commercial
Application
General Description
Cost-effective customized wholesale tert-Butyl (4R-cis)-6-formaldehydel-2,2-dimethyl-1,3-dioxane-4-acetate 124752-23-4
- Molecular Formula:C13H22O5
- Molecular Weight:258.315
- Vapor Pressure:0mmHg at 25°C
- Refractive Index:1.47
- Boiling Point:320.344 °C at 760 mmHg
- Flash Point:140.583 °C
- PSA:61.83000
- Density:1.073 g/cm3
- LogP:1.82730
tert-Butyl (4R-cis)-6-formaldehydel-2,2-dimethyl-1,3-dioxane-4-acetate(Cas 124752-23-4) Usage
InChI:InChI=1/C13H22O5/c1-12(2,3)18-11(15)7-9-6-10(8-14)17-13(4,5)16-9/h8-10H,6-7H2,1-5H3/t9-,10+/m1/s1
124752-23-4 Relevant articles
A new way to tert-Butyl [(4R,6R)-6-aminoethyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate, a key intermediate of atorvastatin synthesis
Radl, Stanislav
, p. 2275 - 2283 (2003)
A new synthesis of tert-butyl [(4R,6R)-6...
Preparation method of rosuvastatin calcium intermediate
-
Paragraph 0033-0049, (2021/07/08)
The invention belongs to the technical f...
Method for preparing rosuvastatin side chain through oxidation in continuous flow micro-channel reactor
-
Paragraph 0042-0089, (2020/08/12)
The invention relates to a method for pr...
Preparation method of rosuvastatin side chain intermediate
-
Paragraph 0047-0063; 0079-0082; 0086-0087, (2020/08/30)
The invention belongs to the technical f...
Rosuvastatin calcium intermediate, preparation method thereof and application of intermediate
-
Paragraph 0083-0085, (2019/04/17)
The invention discloses a rosuvastatin c...
124752-23-4 Process route
-
-
124655-09-0,407577-54-2
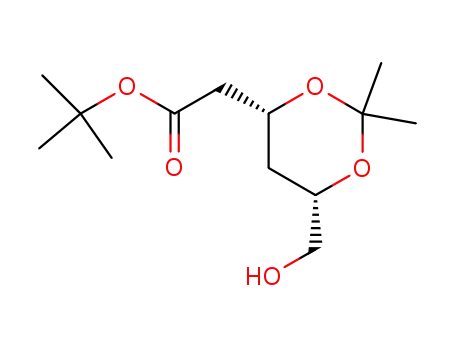
1,1-dimethylethyl (4R-cis)-6-hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-acetate

-
![tert-butyl 2-[(4R,6S)-6-formyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate](/upload/2025/9/244e4ab5-5f29-4c8f-b19f-e93828d18330.png)
-
124752-23-4
tert-butyl 2-[(4R,6S)-6-formyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium hydrogencarbonate; potassium bromide;
In
dichloromethane; water;
at 0 - 5 ℃;
pH=8 - 10;
Temperature;
Solvent;
Inert atmosphere;
|
98% |
|
Swern oxidation; (further oxidizing agents);
|
97% |
|
1,1-dimethylethyl (4R-cis)-6-hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-acetate;
With
dimethyl sulfoxide; N-ethyl-N,N-diisopropylamine;
In
dichloromethane;
at -5 - 0 ℃;
for 0.5h;
With
pyridine; sulfur trioxide pyridine complex; dimethyl sulfoxide;
In
dichloromethane;
at -5 - 20 ℃;
for 1.16667h;
|
93% |
|
With
sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; potassium bromide;
In
dichloromethane; water;
at 5 - 10 ℃;
for 0.166667h;
Temperature;
Flow reactor;
|
93.5% |
|
With
[2,2]bipyridinyl; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; oxygen; copper(l) chloride;
In
dichloromethane; acetonitrile;
at 40 ℃;
|
90% |
|
With
tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide;
In
dichloromethane; acetonitrile;
at 0 - 25 ℃;
for 5.5h;
Molecular sieve;
|
86% |
|
With
2,2,6,6-tetramethyl-piperidine-N-oxyl; sodium hypochlorite; sodium hydrogencarbonate; potassium bromide;
In
dichloromethane;
at -15 - 0 ℃;
pH=10;
Solvent;
Temperature;
Concentration;
|
85.9% |
|
With
sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium hydrogencarbonate; potassium bromide;
In
dichloromethane; water;
at -15 - 5 ℃;
for 1h;
Large scale;
|
83% |
|
With
oxalyl dichloride; dimethyl sulfoxide; triethylamine;
In
dichloromethane;
at -60 - 20 ℃;
|
77% |
|
With
sodium hydrogencarbonate;
|
65% |
|
With
sodium hypochlorite; TEMPOL; sodium hydrogencarbonate; potassium bromide;
In
water; ethyl acetate;
at -10 - 5 ℃;
for 1h;
Inert atmosphere;
|
54% |
|
|
|
|
With
sodium hypochlorite; sodium hydrogencarbonate; potassium bromide;
4-oxo-2,2,6,6-tetramethylpiperidin-oxyl;
In
dichloromethane; water;
at 0 - 5 ℃;
for 1 - 2h;
|
|
|
With
sodium hypochlorite;
2-azatricyclo[3.3.1.13,7]dec-2-yloxidanyl; potassium bromide;
In
dichloromethane;
at -15 - -5 ℃;
|
|
|
1,1-dimethylethyl (4R-cis)-6-hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-acetate;
With
potassium bromide;
2-azatricyclo[3.3.1.13,7]dec-2-yloxidanyl;
In
dichloromethane;
at -15 - -5 ℃;
for 0.25h;
With
sodium hypochlorite;
In
dichloromethane;
at -15 - -5 ℃;
for 0.25h;
|
|
|
1,1-dimethylethyl (4R-cis)-6-hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-acetate;
With
2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium hydrogencarbonate; potassium bromide;
In
dichloromethane;
at 0 - 5 ℃;
for 0.25h;
With
sodium hypochlorite; sodium hydrogencarbonate;
In
dichloromethane; water;
at 0 - 5 ℃;
for 1.66667h;
|
19.2 g |
|
With
[2,2]bipyridinyl; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; oxygen; copper(l) chloride;
In
dichloromethane; acetonitrile;
at 25 - 40 ℃;
Solvent;
|
|
|
1,1-dimethylethyl (4R-cis)-6-hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-acetate;
With
oxalyl dichloride; dimethyl sulfoxide;
In
dichloromethane;
at -78 - 20 ℃;
for 0.25h;
Inert atmosphere;
With
triethylamine;
In
dichloromethane;
at -78 - 20 ℃;
for 4h;
Inert atmosphere;
|
|
|
With
oxalyl dichloride; triethylamine;
In
dichloromethane; dimethyl sulfoxide;
|
|
|
With
N-chloro-succinimide; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; tetrabutylammomium bromide; sodium hydrogencarbonate; potassium carbonate;
In
dichloromethane;
at 10 ℃;
for 5h;
pH=7.5;
Reagent/catalyst;
Solvent;
Temperature;
pH-value;
|
253.6 g |
-
![(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester](/upload/2025/9/50013bf1-02a6-4289-b808-c9954f1945ed.png)
-
154026-95-6
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester

-
![tert-butyl 2-[(4R,6S)-6-formyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate](/upload/2025/9/244e4ab5-5f29-4c8f-b19f-e93828d18330.png)
-
124752-23-4
tert-butyl 2-[(4R,6S)-6-formyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate
| Conditions | Yield |
|---|---|
|
(4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester;
With
methanol; potassium carbonate;
for 3h;
With
sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium hydrogencarbonate; potassium bromide;
In
dichloromethane;
at -5 - 5 ℃;
for 1h;
|
96.1% |
|
Multi-step reaction with 2 steps
1: potassium carbonate; methanol / 0.5 h
2: copper(l) chloride; [2,2]bipyridinyl; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; oxygen / acetonitrile; dichloromethane / 25 - 40 °C
With
methanol; [2,2]bipyridinyl; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; oxygen; potassium carbonate; copper(l) chloride;
In
dichloromethane; acetonitrile;
|
|
|
Multi-step reaction with 2 steps
1: sodium hydroxide / dichloromethane; methanol
2: triethylamine; oxalyl dichloride / dichloromethane; dimethyl sulfoxide
With
oxalyl dichloride; triethylamine; sodium hydroxide;
In
methanol; dichloromethane; dimethyl sulfoxide;
|
124752-23-4 Upstream products
-
124655-09-0

1,1-dimethylethyl (4R-cis)-6-hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-acetate
-
147489-02-9
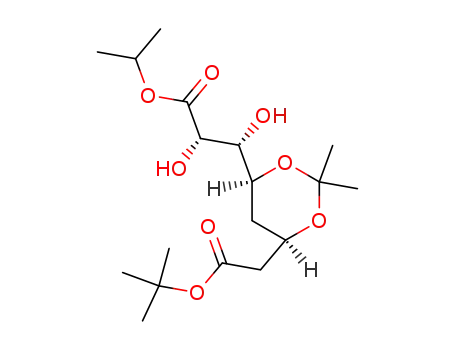
isopropyl (2S,3R,4S,6R)-7-t-butoxycarbonyl-2,3-dihydroxy-4,6-isopropylidenedioxyheptanoate
-
147489-00-7
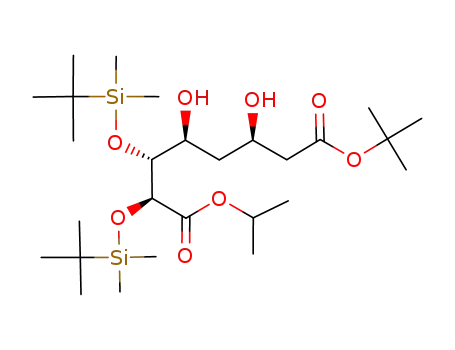
isopropyl (2S,3R,4S,6R)-7-t-butoxycarbonyl-2,3-bis(t-butyldimethylsilyloxy)-4,6-dihydroxyheptanoate
-
147489-01-8
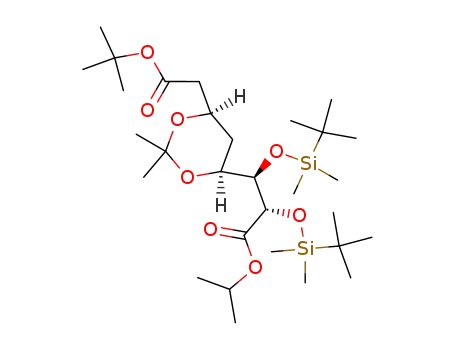
isopropyl (2S,3R,4S,6R)-7-t-butpxycarbonyl-2,3-bis(t-butyldimethylsilyloxy)-4,6-isopropylidenedioxyheptanoate
124752-23-4 Downstream products
-
129894-42-4
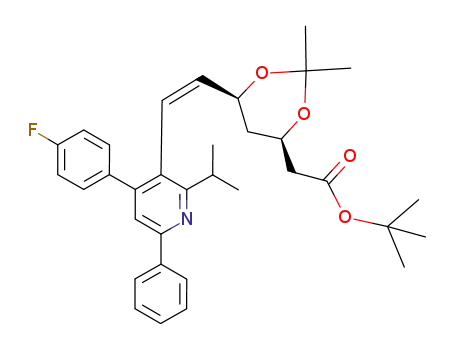
((4R,6S)-6-{(Z)-2-[4-(4-Fluoro-phenyl)-2-isopropyl-6-phenyl-pyridin-3-yl]-vinyl}-2,2-dimethyl-[1,3]dioxan-4-yl)-acetic acid tert-butyl ester
-
124655-11-4
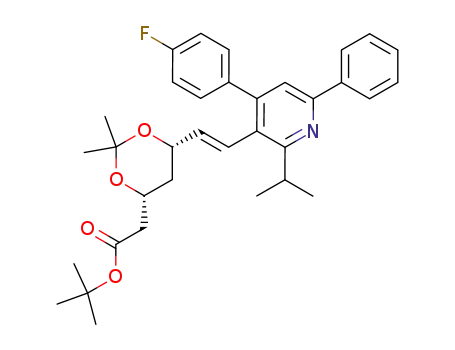
((4R,6S)-6-{(E)-2-[4-(4-Fluoro-phenyl)-2-isopropyl-6-phenyl-pyridin-3-yl]-vinyl}-2,2-dimethyl-[1,3]dioxan-4-yl)-acetic acid tert-butyl ester
-
147489-06-3
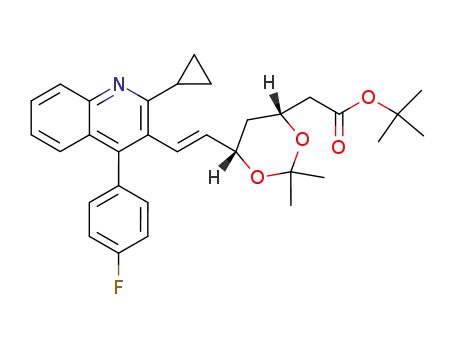
(4R,6S)-(E)-{6-[2-(2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)-vinyl]-2,2-dimethyl-1,3-dioxan-4-yl}acetic acid tert-butyl ester
-
916314-43-7
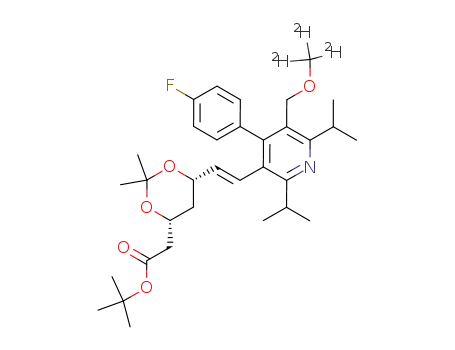
tert-butyl 2-((4R,6S)-6-((E)-2-(4-(4-fluorophenyl)-2,6-diisopropyl-5-[(d3-methoxy)methyl]pyridin-3-yl)vinyl)-2,2-dimethyl-1,3-dioxan-4-yl)acetate


