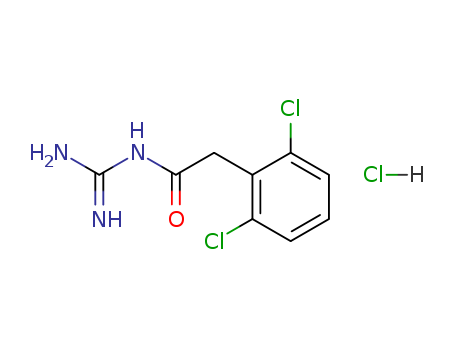
Guanfacine HCL
-
Product Name :
Guanfacine HCL
-
CAS No :
29110-48-3
-
Project State :
Commercial
Application
General Description
High quality purity >99% Guanfacine HCL 29110-48-3 for sale
- Molecular Formula:C9H9 Cl2 N3 O . Cl H
- Molecular Weight:282.557
- Melting Point:215-217℃
- PSA:78.97000
- LogP:3.53850
GUANFACINE HCL(Cas 29110-48-3) Usage
|
Therapeutic Function |
Antihypertensive |
|
Biological Activity |
Selective α 2A -adrenoceptor agonist (K d = 31 nM). Displays 60-fold selectivity over α 2B -adrenoceptors. Also available as part of the α 2 -Adrenoceptor Tocriset? . |
|
Biochem/physiol Actions |
α-2 noradrenergic receptor agonist. |
|
Pharmacokinetics |
The pharmacokinetic properties for guanfacine differ from those of clonidine, guanabenz, and α-methyldopa. At pH 7.4, guanfacine is predominately (67%) in the nonionized, lipid-soluble base form, which accounts for its high oral bioavailability (>80%). Following an oral dose, peak plasma concentrations occur in 1 to 4 hours, with a relatively long elimination half-life of 14 to 23 hours. The maximum blood pressure response occurs in 8 to 12 hours after oral administration and is maintained up to 36 hours following its discontinuation. Following IV dosing, guanfacine achieves the highest concentrations in liver and kidney, with low concentrations in the brain. Guanfacine is 64% bound to plasma proteins. In patients with hepatic or renal impairment, its elimination half-life may be prolonged. Guanfacine is metabolized principally by hepatic hydroxylation to its inactive metabolite, 3-hydroxyguanfacine (20%), which is eliminated in the urine as its glucuronide (30%), sulfate (8%), or mercapturic acid conjugate (10%), and 24 to 37% is excreted as unchanged guanfacine. Its nearly complete bioavailability suggests no evidence of any first-pass effect. Guanfacine and its inactive metabolites are excreted principally in urine, with approximately 80% of its oral dose excreted in urine within 48 hours. |
|
Side effects |
Overall, although the frequency of troublesome adverse effects produced by guanfacine is similar to that produced by clonidine and the other centrally acting sympatholytics, their incidence and severity are lower with guanfacine. Unlike clonidine, abrupt discontinuation of guanfacine rarely results in rebound hypertension. When a withdrawal syndrome has occurred, its onset was slower and its symptoms less severe than the syndrome observed with clonidine. |
|
General Description |
Guanfacine hydrochloride,N-(aminoiminomethyl)-2,6-dichlorobenzeneacetamide(Tenex), is structurally related to clonidine hydrochloride andguanabenz acetate and shares many of their pharmacologicalproperties. The drug has a longer duration of action than eitherclonidine hydrochloride or guanabenz acetate. It lasts upto 24 hours. It also requires much longer (8–12 hours) for apeak effect to occur after the drug is administered. |
InChI:InChI=1/C9H9Cl2N3O.ClH/c10-6-2-1-3-7(11)5(6)4-8(15)14-9(12)13;/h1-3H,4H2,(H4,12,13,14,15);1H
29110-48-3 Relevant articles
THERAPEUTIC COMPOUNDS
-
Paragraph 0538; 0539, (2018/08/30)
The invention provides compounds of form...
The preparation method of the overall tablet sizes hydrochloride (by machine translation)
-
Paragraph 0027; 0028; 0029, (2017/08/29)
The invention discloses a method for pre...
29110-48-3 Process route
-
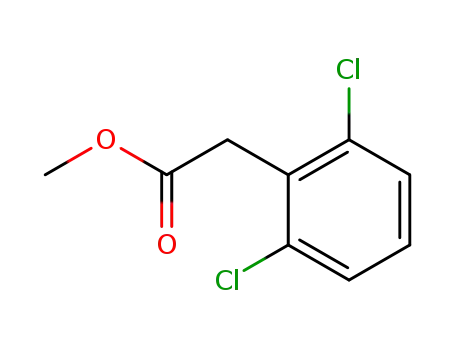
-
54551-83-6
methyl 2-(2,6-dichlorophenyl)acetate

-
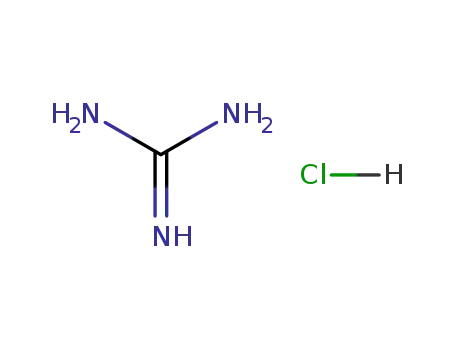
-
50-01-1
guanidine hydrochloride

-
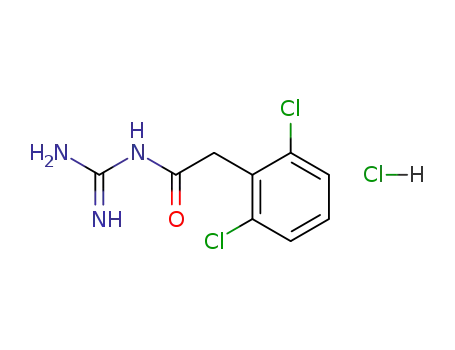
-
29110-48-3,29520-14-7
guanfacine hydrochloride
| Conditions | Yield |
|---|---|
|
In
ethanol;
at 20 ℃;
for 30h;
|
15% |
-
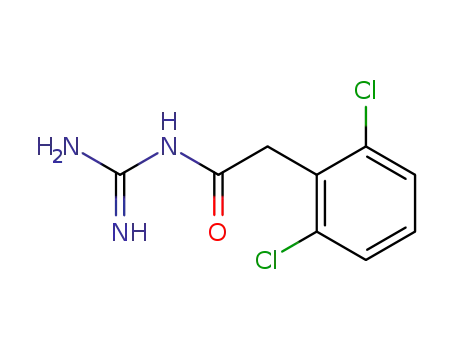
-
29110-47-2
guanfacine

-

-
29110-48-3,29520-14-7
guanfacine hydrochloride
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
ethanol; water;
for 0.5h;
pH=1;
|
32.6 g |
29110-48-3 Upstream products
-
29110-47-2

guanfacine
-
54551-83-6

methyl 2-(2,6-dichlorophenyl)acetate
-
50-01-1

guanidine hydrochloride
29110-48-3 Downstream products
-
1364788-95-3
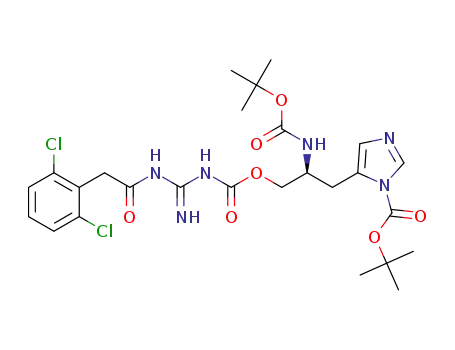
C26H34Cl2N6O7
-
1364788-40-8
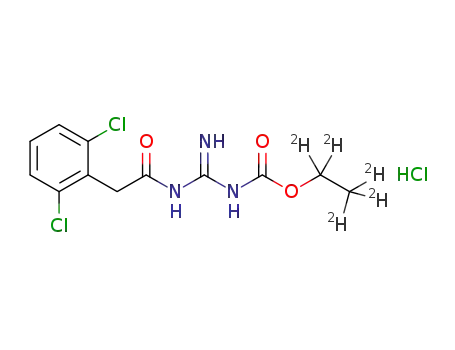
{N'-[2-(2,6-dichloro-phenyl)acetyl]-guanidinocarbonyloxy}ethane-d5 hydrochloride
-
1364788-87-3
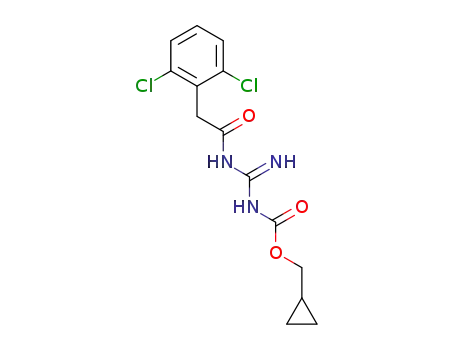
{N'-[2-(2,6-dichloro-phenyl)acetyl]-guanidino-carbonyloxy}methylcyclopropane
-
1364788-84-0
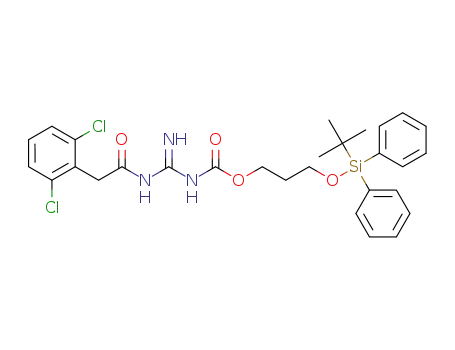
guanfacine [3-(tert-butyl-diphenyl-silanyloxy)-propyl]carbamate


