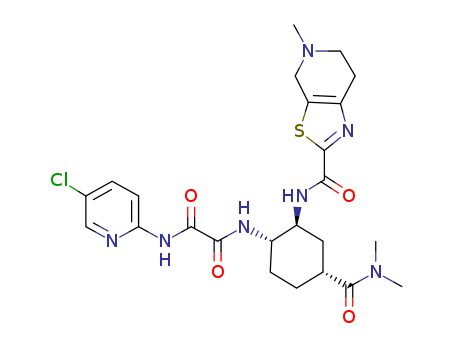
Edoxaban
-
Product Name :
Edoxaban
-
CAS No :
480449-70-5
-
Project State :
Commercial
Application
General Description
Edoxaban 480449-70-5 with purity >99% Low price in stock
- Molecular Formula:C24H30ClN7O4S
- Molecular Weight:548.066
- PKA:9.46±0.70(Predicted)
- PSA:164.87000
- Density:1.43g/cm3
- LogP:2.08230
480449-70-5 Relevant articles
PROCESS FOR PREPARATION OF EDOXABAN
-
Page/Page column 17-18, (2021/01/23)
The present invention relates to process...
Preparation method of high-purity edoxaban
-
Paragraph 0023-0062, (2020/09/12)
The invention belongs to the field of or...
Method for preparing edoxaban from trichloroacetophenone onium salt derivatives
-
Paragraph 0085-0090, (2020/07/21)
The invention provides a method for prep...
Methyltetrahydropyridinothiazole active compound and preparation method and application thereof
-
Paragraph 0092-0107, (2020/11/12)
The invention relates to a methyltetrahy...
480449-70-5 Process route
-
![(1S,3R,4R)-3-[(tert-butoxycarbonyl)amino]-4-hydroxy-N,N-dimethylcyclohexanecarboxamide](/upload/2025/9/fbd9f10a-cee4-427a-a251-c6ca5fc46940.png)
-
929693-30-1
(1S,3R,4R)-3-[(tert-butoxycarbonyl)amino]-4-hydroxy-N,N-dimethylcyclohexanecarboxamide

-
-
480449-70-5
edoxaban
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 5 steps
1.1: triethylamine / 4-methyl-2-pentanone (MIBK) / 1 h / 20 °C
2.1: sodium azide; N-dodecylpyridinium chloride / toluene; water / 47 h / 60 - 63 °C / Dean-Stark
3.1: ammonium formate / palladium-carbon / methanol / 1 h / 40 °C
3.2: 17 h / 20 °C
4.1: triethylamine / acetonitrile / 6 h / 60 °C
5.1: methanesulfonic acid / acetonitrile / 2 h / 20 °C
5.2: 16 h / 20 °C / Cooling with ice
With
sodium azide; methanesulfonic acid; ammonium formate; N-dodecylpyridinium chloride; triethylamine;
palladium-carbon;
In
methanol; 4-methyl-2-pentanone (MIBK); water; toluene; acetonitrile;
|
|
|
Multi-step reaction with 6 steps
1.1: triethylamine / 4-methyl-2-pentanone (MIBK) / 1 h / 20 °C
2.1: sodium azide; N-dodecylpyridinium chloride / ethyl acetate; N,N-dimethylacetamide (DMAC) / 72 h / 20 - 60 °C
3.1: ammonium formate / palladium-carbon / acetonitrile; methanol / 1 h / 40 °C
4.1: acetonitrile; water / 17 h / 20 °C
5.1: triethylamine / acetonitrile / 6 h / 60 °C
6.1: methanesulfonic acid / acetonitrile / 2 h / 20 °C
6.2: 16 h / 20 °C / Cooling with ice
With
sodium azide; methanesulfonic acid; ammonium formate; N-dodecylpyridinium chloride; triethylamine;
palladium-carbon;
In
methanol; N,N-dimethylacetamide (DMAC); 4-methyl-2-pentanone (MIBK); water; ethyl acetate; acetonitrile;
|
|
|
Multi-step reaction with 6 steps
1.1: triethylamine / 4-methyl-2-pentanone (MIBK) / 1 h / 20 °C
2.1: sodium azide; N-dodecylpyridinium chloride / ethyl acetate; N,N-dimethylacetamide (DMAC) / 72 h / 20 - 60 °C
3.1: ammonium formate / palladium-carbon / acetonitrile; methanol / 1 h / 40 °C
4.1: acetonitrile; water / 17 h / 20 °C
5.1: triethylamine / acetonitrile / 7 h / 60 - 75 °C
6.1: methanesulfonic acid / acetonitrile / 2 h / 20 °C
6.2: 16 h / 20 °C / Cooling with ice
With
sodium azide; methanesulfonic acid; ammonium formate; N-dodecylpyridinium chloride; triethylamine;
palladium-carbon;
In
methanol; N,N-dimethylacetamide (DMAC); 4-methyl-2-pentanone (MIBK); water; ethyl acetate; acetonitrile;
|
|
|
Multi-step reaction with 6 steps
1.1: triethylamine / 1 h / 15 - 30 °C
2.1: dodecylpyridinium chloride; sodium azide / toluene / 72 h / 60 °C
3.1: palladium-carbon; ammonium formate / methanol / 1 h / 40 °C
4.1: acetonitrile; water / 17 h / 20 °C
5.1: triethylamine / acetonitrile / 22 h / 20 - 60 °C
6.1: methanesulfonic acid / acetonitrile / 2 h / 20 °C
6.2: 16 h / 20 °C
With
sodium azide; methanesulfonic acid; palladium-carbon; ammonium formate; dodecylpyridinium chloride; triethylamine;
In
methanol; water; toluene; acetonitrile;
|
-
![5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloric acid salt](/upload/2025/9/640e3a27-1a28-48d0-9894-3420bb2367cc.png)
-
720720-96-7
5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloric acid salt

-
![carbamic acid, N-[(1R,2S,5S)-2-[[2-[(5-chloro-2-pyridinyl)amino]-2-oxoacetyl]amino]-5-[(dimethylamino)carbonyl]cyclohexyl]-1,1-dimethylethyl ester](/upload/2025/9/47165f1a-00d7-4a50-a389-d2ded091218e.png)
-
480452-36-6
carbamic acid, N-[(1R,2S,5S)-2-[[2-[(5-chloro-2-pyridinyl)amino]-2-oxoacetyl]amino]-5-[(dimethylamino)carbonyl]cyclohexyl]-1,1-dimethylethyl ester

-

-
480449-70-5
edoxaban
| Conditions | Yield |
|---|---|
|
carbamic acid, N-[(1R,2S,5S)-2-[[2-[(5-chloro-2-pyridinyl)amino]-2-oxoacetyl]amino]-5-[(dimethylamino)carbonyl]cyclohexyl]-1,1-dimethylethyl ester;
With
1-butyl-3-methylimidazolium hydroxide; methanesulfonic acid;
In
acetonitrile;
for 10h;
5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloric acid salt;
With
pyridine; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine;
In
acetonitrile;
at 10 ℃;
for 20h;
Reagent/catalyst;
|
99.69% |
|
carbamic acid, N-[(1R,2S,5S)-2-[[2-[(5-chloro-2-pyridinyl)amino]-2-oxoacetyl]amino]-5-[(dimethylamino)carbonyl]cyclohexyl]-1,1-dimethylethyl ester;
With
methanesulfonic acid;
In
acetonitrile;
at 20 - 60 ℃;
for 3h;
Large scale;
5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloric acid salt;
With
triethylamine; ethyl cyanoglyoxylate-2-oxime; diisopropyl-carbodiimide;
In
acetonitrile;
at 0 - 25 ℃;
for 5h;
Large scale;
|
96.4% |
|
carbamic acid, N-[(1R,2S,5S)-2-[[2-[(5-chloro-2-pyridinyl)amino]-2-oxoacetyl]amino]-5-[(dimethylamino)carbonyl]cyclohexyl]-1,1-dimethylethyl ester;
With
methanesulfonic acid;
In
dichloromethane;
at 20 ℃;
for 2h;
5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloric acid salt;
With
benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine;
In
dichloromethane;
at 20 ℃;
for 18h;
Cooling with ice;
|
85% |
|
carbamic acid, N-[(1R,2S,5S)-2-[[2-[(5-chloro-2-pyridinyl)amino]-2-oxoacetyl]amino]-5-[(dimethylamino)carbonyl]cyclohexyl]-1,1-dimethylethyl ester;
With
hydrogenchloride; water;
In
acetone;
at 0.25 - 0.3 ℃;
for 2h;
5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloric acid salt;
With
benzotriazol-1-ol; N-ethyl-N,N-diisopropylamine; dicyclohexyl-carbodiimide;
In
dichloromethane;
at 0.35 - 0.4 ℃;
for 0.5h;
|
85.13% |
|
carbamic acid, N-[(1R,2S,5S)-2-[[2-[(5-chloro-2-pyridinyl)amino]-2-oxoacetyl]amino]-5-[(dimethylamino)carbonyl]cyclohexyl]-1,1-dimethylethyl ester;
With
methanesulfonic acid;
In
acetonitrile;
at 20 ℃;
for 2h;
5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloric acid salt;
With
benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine;
In
acetonitrile;
at 0 - 20 ℃;
for 17h;
|
|
|
carbamic acid, N-[(1R,2S,5S)-2-[[2-[(5-chloro-2-pyridinyl)amino]-2-oxoacetyl]amino]-5-[(dimethylamino)carbonyl]cyclohexyl]-1,1-dimethylethyl ester;
With
methanesulfonic acid;
In
acetonitrile;
at 20 ℃;
for 2h;
5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloric acid salt;
With
benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine;
In
acetonitrile;
at 20 ℃;
for 16h;
Cooling with ice;
|
|
|
carbamic acid, N-[(1R,2S,5S)-2-[[2-[(5-chloro-2-pyridinyl)amino]-2-oxoacetyl]amino]-5-[(dimethylamino)carbonyl]cyclohexyl]-1,1-dimethylethyl ester;
With
methanesulfonic acid;
In
acetonitrile;
at 20 ℃;
for 2h;
5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloric acid salt;
With
benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine;
In
acetonitrile;
at 20 ℃;
for 16h;
Cooling with ice;
|
|
|
carbamic acid, N-[(1R,2S,5S)-2-[[2-[(5-chloro-2-pyridinyl)amino]-2-oxoacetyl]amino]-5-[(dimethylamino)carbonyl]cyclohexyl]-1,1-dimethylethyl ester;
With
methanesulfonic acid;
In
acetonitrile;
at 20 ℃;
for 2h;
5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloric acid salt;
With
benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine;
In
acetonitrile;
at 20 ℃;
for 16h;
Cooling with ice;
|
103.2 g |
|
carbamic acid, N-[(1R,2S,5S)-2-[[2-[(5-chloro-2-pyridinyl)amino]-2-oxoacetyl]amino]-5-[(dimethylamino)carbonyl]cyclohexyl]-1,1-dimethylethyl ester;
With
methanesulfonic acid;
In
acetonitrile;
at 20 ℃;
for 2h;
5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloric acid salt;
With
benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine;
In
acetonitrile;
at 20 ℃;
for 17h;
Cooling with ice;
|
103.2 g |
|
carbamic acid, N-[(1R,2S,5S)-2-[[2-[(5-chloro-2-pyridinyl)amino]-2-oxoacetyl]amino]-5-[(dimethylamino)carbonyl]cyclohexyl]-1,1-dimethylethyl ester;
With
methanesulfonic acid;
In
acetonitrile;
at 20 ℃;
for 2h;
5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloric acid salt;
With
1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine;
In
acetonitrile;
at 20 ℃;
for 16h;
|
103.2 g |
|
carbamic acid, N-[(1R,2S,5S)-2-[[2-[(5-chloro-2-pyridinyl)amino]-2-oxoacetyl]amino]-5-[(dimethylamino)carbonyl]cyclohexyl]-1,1-dimethylethyl ester;
With
methanesulfonic acid;
In
acetonitrile;
at 20 ℃;
for 2h;
5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloric acid salt;
With
benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine;
In
water; acetonitrile;
at 20 ℃;
for 17h;
|
103.2 g |
|
carbamic acid, N-[(1R,2S,5S)-2-[[2-[(5-chloro-2-pyridinyl)amino]-2-oxoacetyl]amino]-5-[(dimethylamino)carbonyl]cyclohexyl]-1,1-dimethylethyl ester;
With
methanesulfonic acid;
In
acetonitrile;
at 25 ℃;
for 2h;
With
triethylamine;
In
acetonitrile;
at 10 ℃;
for 0.166667h;
5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloric acid salt;
With
benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;
In
acetonitrile;
at 25 ℃;
for 18h;
|
125.3 g |
|
carbamic acid, N-[(1R,2S,5S)-2-[[2-[(5-chloro-2-pyridinyl)amino]-2-oxoacetyl]amino]-5-[(dimethylamino)carbonyl]cyclohexyl]-1,1-dimethylethyl ester;
With
methanesulfonic acid;
In
acetonitrile;
at 20 ℃;
for 2h;
5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloric acid salt;
With
triethylamine;
In
acetonitrile;
at 20 ℃;
for 16h;
Cooling with ice;
|
103.2 g |
|
carbamic acid, N-[(1R,2S,5S)-2-[[2-[(5-chloro-2-pyridinyl)amino]-2-oxoacetyl]amino]-5-[(dimethylamino)carbonyl]cyclohexyl]-1,1-dimethylethyl ester;
With
methanesulfonic acid;
In
acetonitrile;
at 20 ℃;
for 2h;
5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloric acid salt;
With
benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine;
In
acetonitrile;
at 20 ℃;
for 16h;
Temperature;
Cooling with ice;
|
103.2 g |
480449-70-5 Upstream products
-
480452-37-7
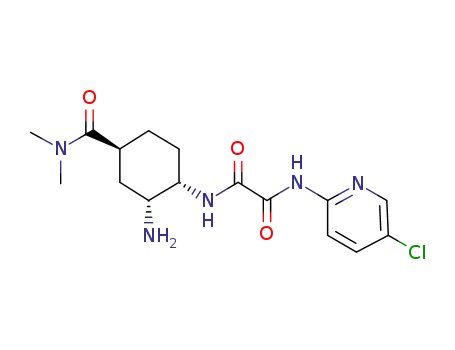
N1-[(1S,2R,4S)-2-amino-4-[(dimethylamino)carbonyl]cyclohexyl]-N2-(5-chloro-2-pyridyl)ethanediamide
-
720720-96-7
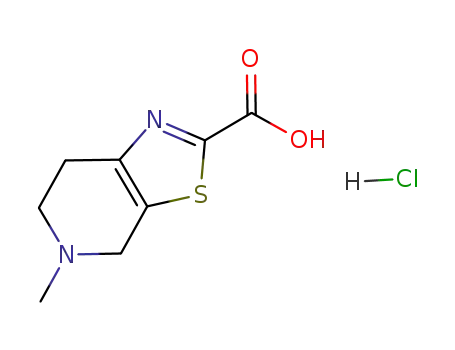
5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloric acid salt
-
480452-36-6
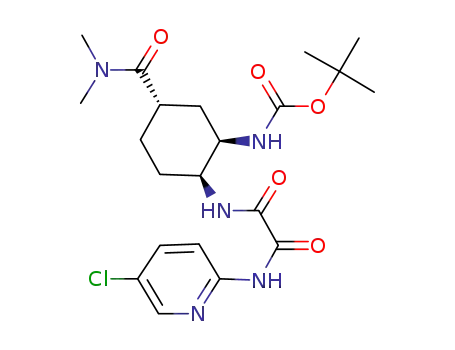
carbamic acid, N-[(1R,2S,5S)-2-[[2-[(5-chloro-2-pyridinyl)amino]-2-oxoacetyl]amino]-5-[(dimethylamino)carbonyl]cyclohexyl]-1,1-dimethylethyl ester
-
480450-69-9
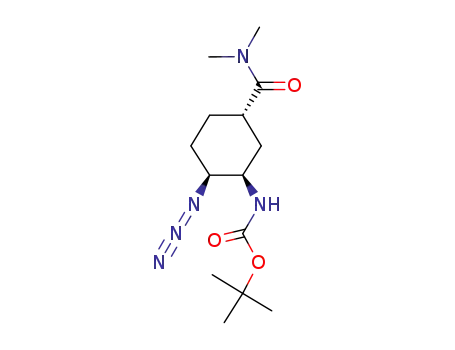
tert-butyl N-[(1R,2S,5S)-2-azido-5-[(dimethylamino)carbonyl]cyclohexyl]carbamate
480449-70-5 Downstream products
-
480449-71-6

[14C]-Edoxaban tosylate


