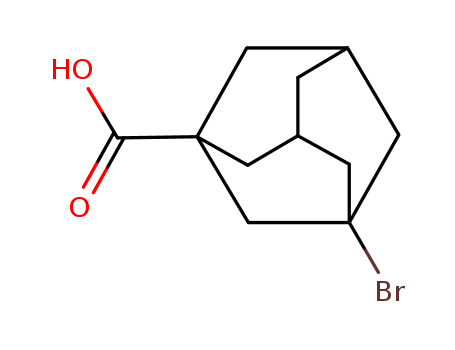
3-Bromo-1-adamantanecarboxylic acid
-
Product Name :
3-Bromo-1-adamantanecarboxylic acid
-
CAS No :
21816-08-0
-
Project State :
Commercial
Application
General Description
High quality purity >99% 3-Bromo-1-adamantanecarboxylic acid 21816-08-0 for sale
- Molecular Formula:C11H15BrO2
- Molecular Weight:259.143
- Vapor Pressure:5.69E-06mmHg at 25°C
- Melting Point:147 °C
- Boiling Point:354.2 °C at 760 mmHg
- PKA:4.35±0.40(Predicted)
- Flash Point:168 °C
- PSA:37.30000
- Density:1.665 g/cm3
- LogP:2.80490
3-Bromoadamantane-1-carboxylic acid(Cas 21816-08-0) Usage
|
Purification Methods |
Purify the acid by recrystallising it from cyclohexane and/or subliming at 130o/10mm. It is converted to the methyl ester (diazomethane) with m 32o (from pet ether at -10o). [Stetter & Mayer Chem Ber 95 667 1962, Stetter & Wulff Chem Ber 93 1366 1960, Bayal & Lantvoev J Org Chem USSR (Engl Trans) 9 291 1973.] |
InChI:InChI=1/C11H15BrO2/c12-11-4-7-1-8(5-11)3-10(2-7,6-11)9(13)14/h7-8H,1-6H2,(H,13,14)/p-1/t7-,8-,10?,11?/m1/s1
21816-08-0 Relevant articles
Investigation of the gas phase reactivity of the 1-adamantyl radical using a distonic radical anion approach
Harman, David G.,Blanksby, Stephen J.
, p. 3495 - 3503 (2007)
The gas phase reactions of the bridgehea...
Disubstituted adamantyl derivative or pharmaceutically acceptable salt thereof, and pharmaceutical composition and kit for inhibiting the growth of cancer containing the same as an active ingredient
-
Paragraph 0212-0214; 0232-0236, (2021/07/20)
The present invention relates to: a disu...
METHODS OF INDUCING AN ANTI-CANCER IMMUNE RESPONSE
-
Page/Page column 36-37, (2020/07/31)
A method or preparing immunologically pr...
Synthesis method of 3-amino-1-adamantanol
-
Paragraph 0014-0015, (2020/06/16)
The invention discloses a synthesis meth...
Azido-Adamantyl Tin Sulfide Clusters for Bioconjugation
Berndt, Jan-Philipp,Engel, Annikka,Hrdina, Radim,Dehnen, Stefanie,Schreiner, Peter R.
, p. 329 - 335 (2019/02/01)
We present a new versatile route toward ...
21816-08-0 Process route
-
-
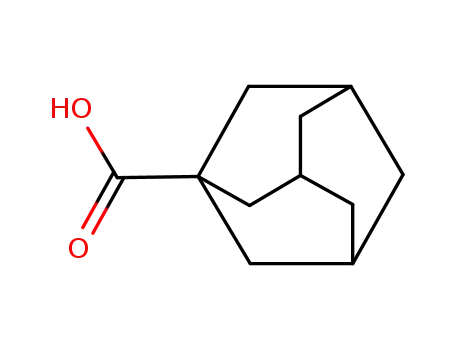
828-51-3
1-Adamantanecarboxylic acid

-
-
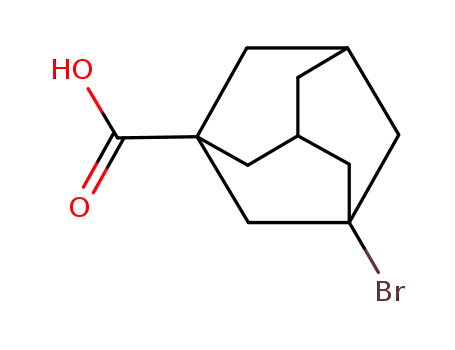
21816-08-0
3-bromoadamantane-1-carboxylic acid
| Conditions | Yield |
|---|---|
|
With
bromine; acetic acid;
for 19h;
Product distribution;
var. reaction time and reagents; other adamantane-1-carboxylic acid derivatives;
|
96% |
|
With
bromine; acetic acid;
for 19h;
Heating;
|
96% |
|
With
aluminum (III) chloride; bromine;
at -5 - 20 ℃;
|
93% |
|
With
aluminum (III) chloride; bromine;
In
dichloromethane;
at -5 - 20 ℃;
for 49h;
Inert atmosphere;
|
93% |
|
With
aluminum (III) chloride; bromine;
at -5 - 20 ℃;
for 49h;
Inert atmosphere;
|
92.83% |
|
With
aluminum tri-bromide;
|
81% |
|
With
aluminum (III) chloride; bromine;
at 0 - 20 ℃;
for 53h;
|
75.7% |
|
With
aluminum (III) chloride; bromine;
at 0 - 20 ℃;
for 53h;
|
75.7% |
|
With
bromine;
at 0 - 20 ℃;
for 53h;
|
75.7% |
|
With
bromine;
for 6h;
Reflux;
|
62% |
|
With
aluminum tri-bromide; bromine;
|
|
|
With
bromine;
In
tetrachloromethane;
|
|
|
Multi-step reaction with 2 steps
1: nitric acid; sulfuric acid / 5 h / 0 °C
2: hydrogen bromide / water / 4.5 h / 90 °C
With
sulfuric acid; hydrogen bromide; nitric acid;
In
water;
|
|
|
With
aluminum (III) chloride; bromine;
at 10 - 30 ℃;
|
-

-
42711-75-1
3-hydroxyadamantane-1-carboxylic acid

-

-
21816-08-0
3-bromoadamantane-1-carboxylic acid
| Conditions | Yield |
|---|---|
|
With
hydrogen bromide;
at 90 ℃;
for 3.5h;
|
99.8% |
|
With
hydrogen bromide;
In
water;
at 90 ℃;
for 4.5h;
|
92% |
21816-08-0 Upstream products
-
828-51-3

1-Adamantanecarboxylic acid
-
42711-78-4
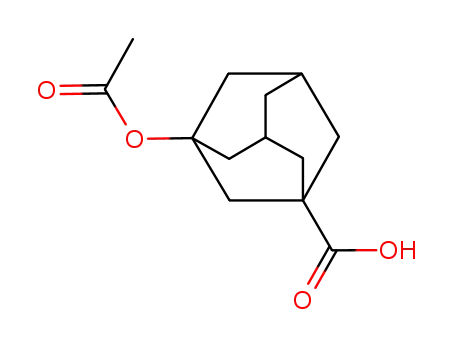
3-acetoxyadamantane-1-carboxylic acid
-
50795-82-9
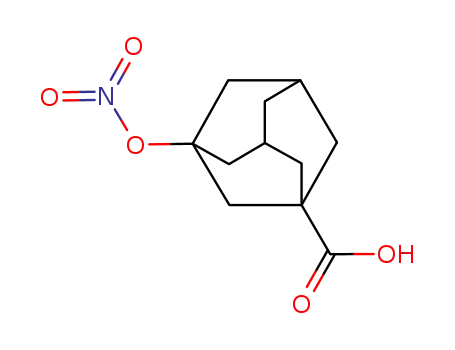
3-nitroxyadamantane-1-carboxylic acid
-
42711-75-1

3-hydroxyadamantane-1-carboxylic acid
21816-08-0 Downstream products
-
53263-89-1
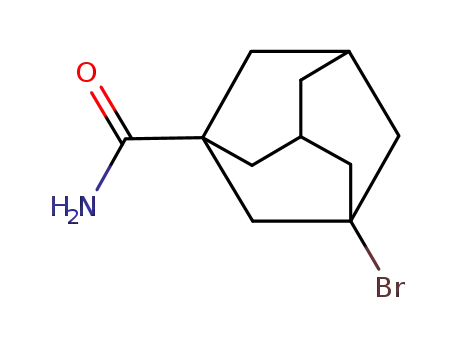
1-bromo-3-carboxamidoadamantane
-
36964-33-7
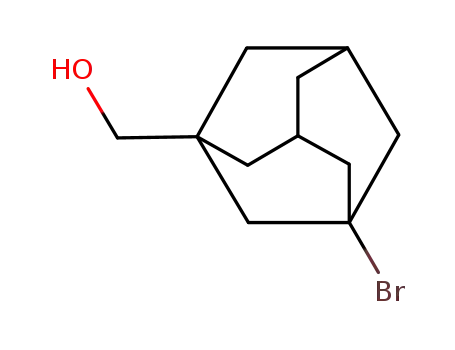
(3-bromotricyclo[3.3.1.13,7]dec-1-yl)methanol
-
39917-36-7
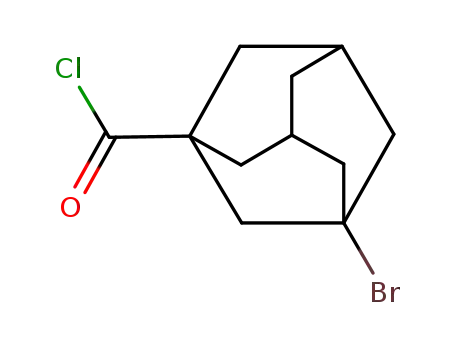
3-bromo-1-adamantanecarbonyl chloride
-
42711-75-1

3-hydroxyadamantane-1-carboxylic acid


