o-Toluic acid
-
Product Name :
o-Toluic acid
-
CAS No :
118-90-1
-
Project State :
Commercial

Application
General Description
Good factory exports good o-Toluic acid 118-90-1
- Molecular Formula:C8H8O2
- Molecular Weight:136.15
- Appearance/Colour:Pale yellow crystals or off-white flaky solid.
- Vapor Pressure:0.00603mmHg at 25°C
- Melting Point:103-105 ºC
- Refractive Index:1.512
- Boiling Point:260.9 ºC at 760 mmHg
- PKA:3.91(at 25℃)
- Flash Point:118.8 ºC
- PSA:37.30000
- Density:1.151 g/cm3
- LogP:1.69320
o-Toluic acid(Cas 118-90-1) Usage
|
Synthesis Reference(s) |
Organic Syntheses, Coll. Vol. 2, p. 588, 1943The Journal of Organic Chemistry, 25, p. 616, 1960 DOI: 10.1021/jo01074a035Tetrahedron Letters, 22, p. 1013, 1981 DOI: 10.1016/S0040-4039(01)82853-7 |
|
Air & Water Reactions |
Fine dust dispensed in air in sufficient concentrations, and in the presence of an ignition source is a potential dust explosion hazard. . Insoluble in water. |
|
Reactivity Profile |
o-Toluic acid is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in o-Toluic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions. o-Toluic acid is incompatible with strong oxidizers. |
|
Fire Hazard |
Flash point data for o-Toluic acid are not available. o-Toluic acid is probably combustible. Fine dust dispensed in air in sufficient concentrations, and in the presence of an ignition source is a potential dust explosion hazard. |
|
Purification Methods |
Crystallise the acid from *benzene (2.5mL/g) and dry in air. The S-benzylisothiuronium salt has m 146o (from aqueous EtOH). [Beilstein 9 IV 1697.] |
|
Definition |
ChEBI: A methylbenzoic acid that is benzoic acid substituted by a methyl group at position 2. |
InChI:InChI=1/C8H8O2/c1-6-4-2-3-5-7(6)8(9)10/h2-5H,1H3,(H,9,10)/p-1
118-90-1 Relevant articles
Surfactant-assisted assembly of nanoscale zinc coordination compounds to enhance tandem conversion reactions in water
Huang, Chao,Zhu, Kaifang,Zhang, Yingying,Lu, Guizhen,Shao, Zhichao,Gao, Kuan,Mi, Liwei,Hou, Hongwei
, p. 16008 - 16016 (2019)
Precise control over the morphology and ...
Steric effect of substituents in haloarenes on the rate of cross-coupling reactions
Khaibulova, T. Sh.,Boyarskaya,Boyarskii
, p. 360 - 365 (2013)
The relative reactivity of ortho- and pa...
Experimental and theoretical investigation of the oxidative carbonylation of toluene to toluic acid catalyzed by palladium(II) in the presence of vanadium and molecular oxygen
Behn, Andrew,Zakzeski, Joseph,Head-Gordon, Martin,Bell, Alexis T.
, p. 91 - 97 (2012)
The mechanism and kinetics of the liquid...
ARYL RADICALS FROM ORGANOMETALLIC SOURCES. PREFERENTIAL ATTACK ON THE SIDE CHAIN OF TOLUENES BY THE o-TOLYL RADICALS.
Battaglia, Luigi Pietro,Nardelli, Mario,Pelizzi, Corrado,Predieri, Giovanni,Chiusoli, Gian Paolo
, p. C19 - C22 (1986)
The o-tolyl radicals, formed by the reac...
Cetyltrimethylammonium dichromate: A phase-transferring oxidant
Patel, Sabita,Kuanar, Minati,Nayak, Biswa B.,Banichul,Mishra, Bijay K.
, p. 1033 - 1037 (2005)
A phase-transferring oxidant, cetyltrime...
An efficient approach for the deprotection of esters using ionic liquid as nucleophile
Wei, Benmei,Zhang, Zhiyong,Dai, Zhiqun,Guan, Jintao
, p. 6404 - 6406 (2013)
An efficient approach for the deprotecti...
Electrochemical reduction of phthalide at carbon cathodes in dimethylformamide: Effects of supporting electrolyte and gas chromatographic injector-port chemistry on the product distribution
Pasciak, Erick M.,Hochstetler, Spencer E.,Mubarak, Mohammad S.,Evans, Dennis H.,Peters, Dennis G.
, p. 557 - 563 (2013)
Cyclic voltammetry and controlled-potent...
Co and Mn polysiloxanes as unique initiator-catalyst-systems for the selective liquid phase oxidation of o-xylene
Foerster, Tobias,Schunk, Stephan A.,Jentys, Andreas,Lercher, Johannes A.
, p. 3254 - 3256 (2011)
Co and Mn polysiloxanes are unique catal...
Pd(OAc)2 promoted bis-N-heterocyclic carbene-catalyzed oxidative transformation of aldehydes
Yu, Ya-Han,Wang, Tsui,Chiu, Chien-Cheng,Lu, Ta-Jung,Lee, Dong-Sheng
, p. 202 - 205 (2020)
The bis-N-heterocyclic carbene-catalyzed...
Application of chloroaluminate ionic liquid as catalyst and medium for the dealkylation of esters
Wei, Ben-Mei,Zhang, Zhi-Yong,Dai, Zhi-Qun,Zhang, Kai-Cheng
, p. 1029 - 1033 (2011)
Dealkylation of esters to carboxylic aci...
Selective liquid phase oxidation of o-xylene with gaseous oxygen by transition metal containing polysiloxane initiator/catalyst systems
Foerster, Tobias,Schunk, Stephan A.,Jentys, Andreas,Lercher, Johannes A.
, p. 25 - 33 (2011)
The selective liquid phase oxidation of ...
Synthesis of anthracene-9-C114.
STEVENS,HOLLAND
, p. 718 - 719 (1950)
-
Formation of a Cluster H2V10O 284– under the Action of Br?nsted Acids and Its Catalytic Activity in Oxidation of Alkylbenzenes
Ul’yanova,Pervova,Slepukhin,Aksenova,Pestov
, p. 687 - 690 (2018)
New method was developed for the prepara...
Flow carbonylation of sterically hindered ortho-substituted iodoarenes
Mallia, Carl J.,Walter, Gary C.,Baxendale, Ian R.
, p. 1503 - 1511 (2016)
The flow synthesis of ortho-substituted ...
Functionalization of benzylic C(sp3)-H bonds of heteroaryl aldehydes through N-Heterocyclic carbene organocatalysis
Chen, Xingkuan,Yang, Song,Song, Bao-An,Chi, Yonggui Robin
, p. 11134 - 11137 (2013)
Aryl aldehyde activation: Oxidative acti...
-
Fichter,Rinderspacher
, p. 40 (1927)
-
Beneficial effect of TMSCl in the Lewis acid-mediated carboxylation of aromatic compounds with carbon dioxide
Nemoto, Koji,Yoshida, Hiroki,Suzuki, Yutaka,Morohashi, Naoya,Hattori, Tetsutaro
, p. 820 - 821 (2006)
The Lewis acid-mediated carboxylation of...
Palladium(II)-catalyzed sequential hydroxylation-carboxylation of biphenyl using formic acid as a carbonyl source
Shibahara, Fumitoshi,Kinoshita, Shinsuke,Nozaki, Kyoko
, p. 2437 - 2439 (2004)
(Equation Presented) A simultaneous hydr...
Thermal reactions of benzocyclobutenone with alcohols
Wang, Zhi Yuan,Suzzarini, Laurence,Gao, Jian Ping
, p. 5745 - 5746 (1997)
Thermolysis of benzocyclobutenone alone ...
Practical preparation of benzyloxyacetic acids
Linn, Kathleen,Kuethe, Jeffrey T.,Peng, Zhihui,Yasuda, Nobuyoshi
, p. 3762 - 3765 (2008)
An efficient and practical method for th...
-
Fisher,Grant
, p. 718 (1935)
-
-
Austin et al.
, p. 864 (1937)
-
Photolysis of a series of α-brominated ortho-xylenes in apolar solvents
Rezende, Daisy de B.,Campos, Ivan P. de Arruda,Toscano, Vicente G.,Catalani, Luiz H.
, p. 1857 - 1862 (1995)
The α-brominated ortho-xylenes have been...
Rapid microwaves synthesis of CoSix/CNTs as novel catalytic materials for hydrogenation of phthalic anhydride
Zhang, Liangliang,Chen, Xiao,Jin, Shaohua,Guan, Jingchao,Williams, Christopher T.,Peng, Zhijian,Liang, Changhai
, p. 105 - 112 (2014)
CoSix/CNTs catalysts with different CoSi...
-
Marvell,E.N.,Sexton,H.
, p. 2919 - 2922 (1964)
-
Direct carboxylation of arenes and halobenzenes with CO2 by the combined use of AlBr3 and R3SiCl
Nemoto, Koji,Yoshida, Hiroki,Egusa, Naoki,Morohashi, Naoya,Hattori, Tetsutaro
, p. 7855 - 7862 (2010)
The Lewis acid-mediated direct carboxyla...
-
Wallace et al.
, p. 2907 (1964)
-
Nanosheet-assembled microflower-like coordination polymers by surfactant-assisted assembly with enhanced catalytic activity
Han, Suzhen,Hu, Mingjun,Huang, Chao,Lu, Guizhen,Mi, Liwei,Qin, Na,Zhang, Ying-Ying,Zhu, Kaifang
, p. 7858 - 7863 (2020)
Tuning the morphology and size of coordi...
-
Ferguson,Wims
, p. 668,669 (1960)
-
-
Brill
, p. 837,838 (1960)
-
Mesoporous Metal Oxide Encapsulated Gold Nanocatalysts: Enhanced Activity for Catalyst Application to Solvent-Free Aerobic Oxidation of Hydrocarbons
Liu, Yali,Gao, Tu-Nan,Chen, Xi,Li, Kaiqian,Ma, Yali,Xiong, Hailong,Qiao, Zhen-An
, p. 12953 - 12960 (2018)
Here, we present a series of experimenta...
The Carboxylic Acid Group as an Effective Director of Ortho-Lithiation
Mortier, Jacques,Moyroud, Joeel,Bennetau, Bernard,Cain, Paul A.
, p. 4042 - 4044 (1994)
Treatment of PhCO2H in tetrahydrofuran w...
-
Hauser,Hoffenberg
, p. 1448,1449 (1955)
-
Ultrasmall Platinum Nanoparticles Supported Inside the Nanospaces of Periodic Mesoporous Organosilica with an Imidazolium Network: An Efficient Catalyst for the Aerobic Oxidation of Unactivated Alcohols in Water
Karimi, Babak,Naderi, Zahra,Khorasani, Mojtaba,Mirzaei, Hamid M.,Vali, Hojatollah
, p. 906 - 910 (2016)
The imidazolium group inside the wall of...
An efficient Pd(II)-based catalyst system for carboxylation of aromatic C-H bond by addition of a phosphenium salt
Sakakibara, Ken,Yamashita, Makoto,Nozaki, Kyoko
, p. 959 - 962 (2005)
Addition of a phosphenium dramatically i...
The synthesis of C14 carbonyl labeled dimethyl phthalate.
BARKER,CHRISTIAN
, p. 105 - 107 (1955)
-
Mechanochemical Grignard Reactions with Gaseous CO2 and Sodium Methyl Carbonate**
Pfennig, Victoria S.,Villella, Romina C.,Nikodemus, Julia,Bolm, Carsten
supporting information, (2022/01/22)
A one-pot, three-step protocol for the p...
Gram-scale synthesis of carboxylic acids via catalytic acceptorless dehydrogenative coupling of alcohols and hydroxides at an ultralow Ru loading
Chen, Cheng,Cheng, Hua,Verpoort, Francis,Wang, Zhi-Qin,Wu, Zhe,Yuan, Ye,Zheng, Zhong-Hui
, (2021/12/13)
Acceptorless dehydrogenative coupling (A...
Ru-Catalyzed C(sp2)?H Bond Arylation of Benzamides Bearing a Novel 4-Aminoantipyrine as a Directing Group
Al Mamari, Hamad H.,Al Kiumi, Diana,Al Rashdi, Tamadher,Al Quraini, Huda,Al Rashdi, Malak,Al Sheraiqi, Sumayya,Al Harmali, Sara,Al Lamki, Mohammed,Al Sheidi, Ahmed,Al Zadjali, Asma
, p. 3598 - 3603 (2021/07/22)
A novel design-based removable N,O-biden...
Photo-tunable oxidation of toluene and its derivatives catalyzed by TBATB
Mardani, Atefeh,Kazemi, Foad,Kaboudin, Babak
, (2021/05/04)
In this report, tetrabutylammonium tribr...
118-90-1 Process route
-
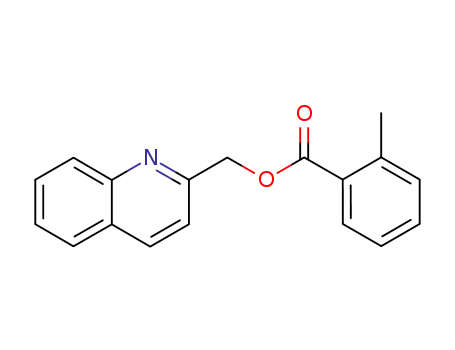
-
77934-71-5
2-Methyl-benzoic acid quinolin-2-ylmethyl ester

-
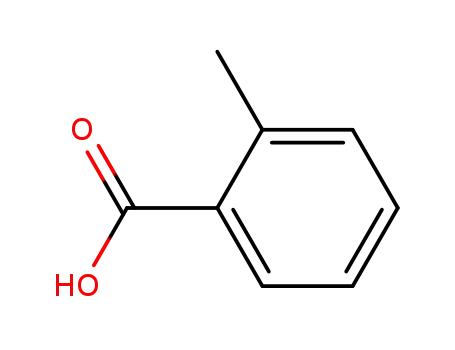
-
118-90-1
ortho-methylbenzoic acid

-
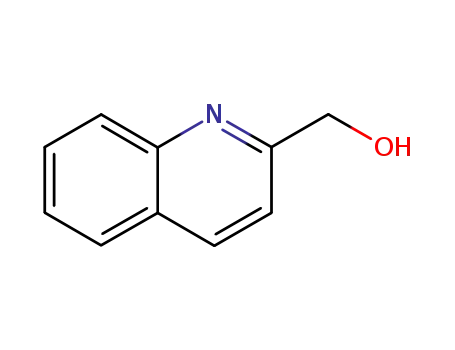
-
1780-17-2
quinolin-2-ylmethanol
| Conditions | Yield |
|---|---|
|
With
water in alkaline medium;
In
acetone;
Rate constant;
Ambient temperature;
|
-
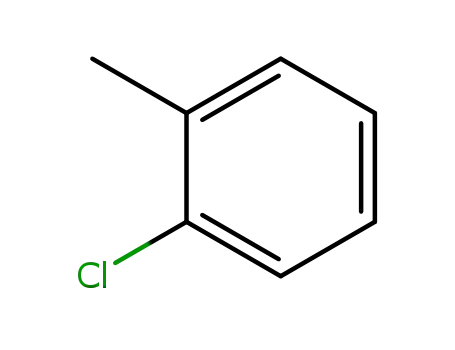
-
95-49-8
2-methylchlorobenzene

-
-
3034-79-5
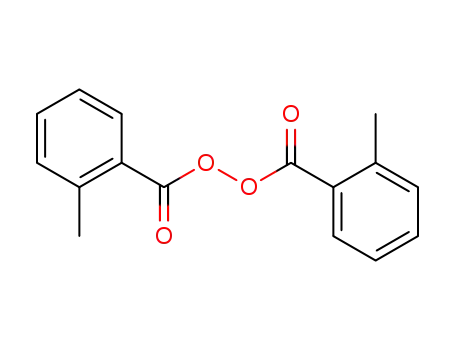
bis(2-methylbenzoyl) peroxide

-

-
118-90-1
ortho-methylbenzoic acid

-
-
87-41-2
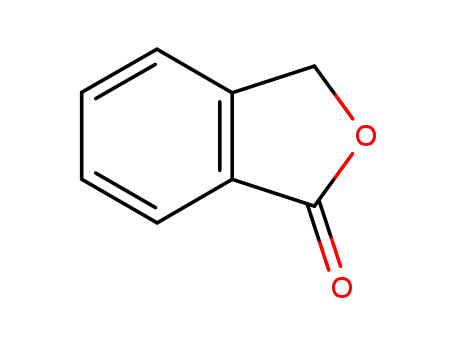
2-benzofuran-1(3H)-one

-
-
17849-38-6
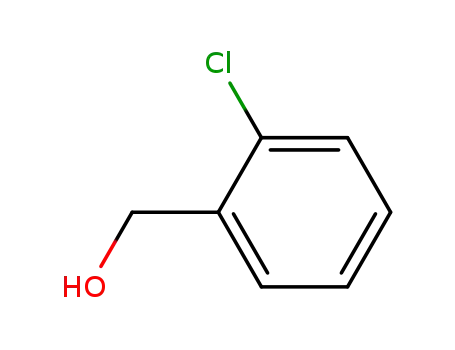
2-Chlorobenzyl alcohol

-
-
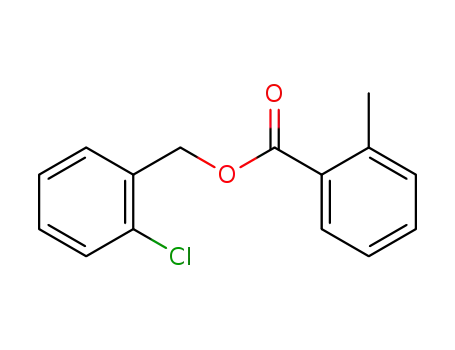
23597-23-1
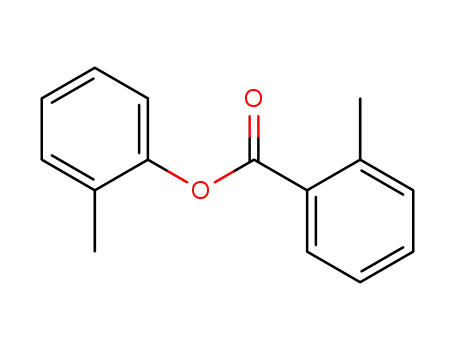
2-methyl-benzoic acid o-tolyl ester

-
-
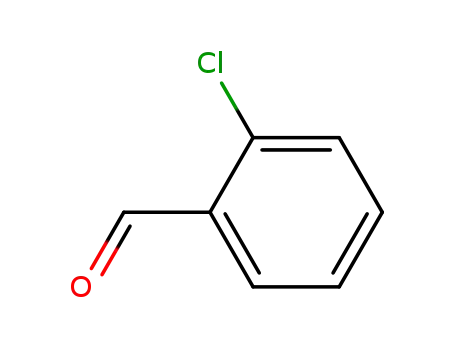
89-98-5
2-chloro-benzaldehyde

-
-
o-chlorobenzyl o-toluate
| Conditions | Yield |
|---|---|
|
With
air;
at 90 ℃;
for 48h;
Product distribution;
|
118-90-1 Upstream products
-
932-31-0
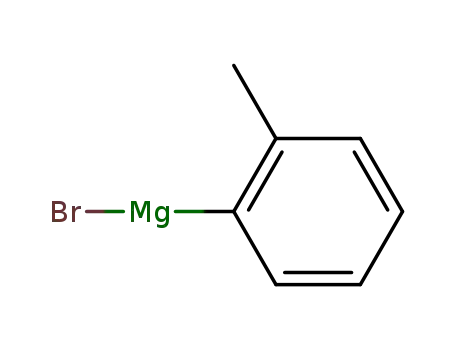
ortho-tolylmagnesium bromide
-
87-41-2

2-benzofuran-1(3H)-one
-
85-44-9
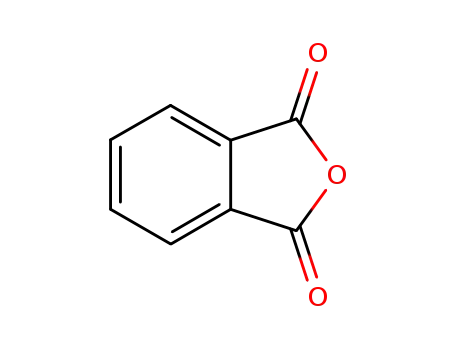
phthalic anhydride
-
2289-03-4
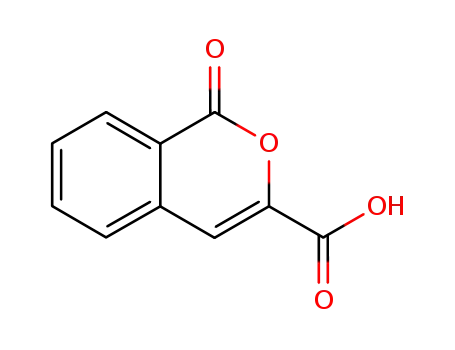
1-oxo-1H-isochromene-3-carboxylic acid
118-90-1 Downstream products
-
89-71-4
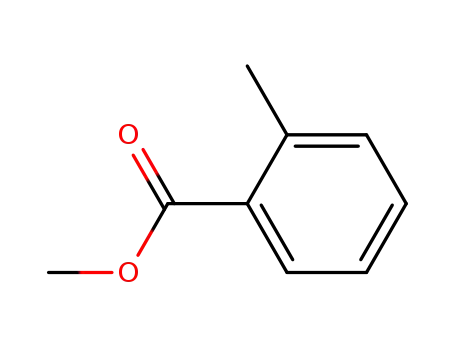
2-Methyl-benzoic acid methyl ester
-
58138-81-1
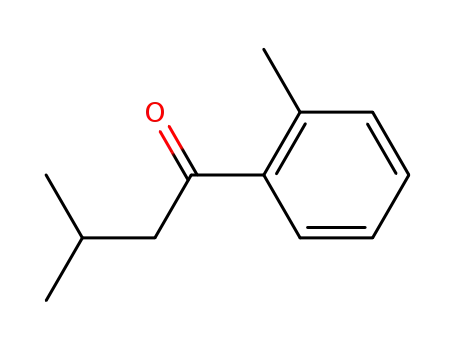
3-methyl-1-(o-tolyl)butan-1-one
-
16216-13-0
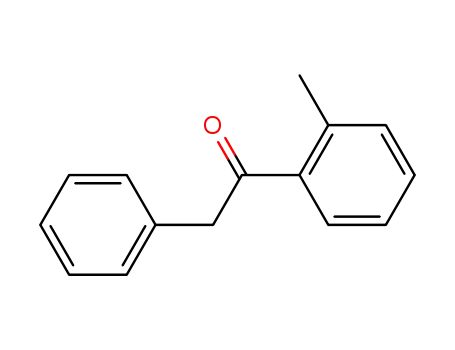
2-phenyl-1-o-tolylethanone
-
95280-74-3
6,6'-dimethyl-3,3'-methanediyl-di-benzoic acid


