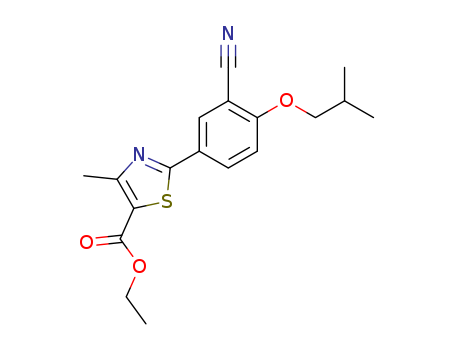
Ethyl 2-(3-cyano-4-isobutoxyphenyl)-4-methyl-5-thiazolecarboxylate
-
Product Name :
Ethyl 2-(3-cyano-4-isobutoxyphenyl)-4-methyl-5-thiazolecarboxylate
-
CAS No :
160844-75-7
-
Project State :
Commercial
Application
General Description
Reliable Quality Ethyl 2-(3-cyano-4-isobutoxyphenyl)-4-methyl-5-thiazolecarboxylate 160844-75-7 Hot Sale with Chinese Manufacturer
- Molecular Formula:C18H20N2O3S
- Molecular Weight:344.434
- Vapor Pressure:0mmHg at 25°C
- Melting Point:176 °C
- Refractive Index:1.57
- Boiling Point:497.149 °C at 760 mmHg
- PKA:1.02±0.10(Predicted)
- Flash Point:254.467 °C
- PSA:100.45000
- Density:1.22 g/cm3
- LogP:4.20168
Ethyl 2-(3-cyano-4-isobutoxyphenyl)-4-methyl-5-thiazolecarboxylate(Cas 160844-75-7) Usage
InChI:InChI=1/C18H20N2O3S/c1-5-22-18(21)16-12(4)20-17(24-16)13-6-7-15(14(8-13)9-19)23-10-11(2)3/h6-8,11H,5,10H2,1-4H3
160844-75-7 Relevant articles
Synthesis, structural elucidation and larvicidal activity of novel arylhydrazones
P, Nefisath,Dasappa, Jagadeesh Prasad,B, Haripriya,Chopra, Deepak,Venugopala, Katharigatta N.,Deb, Pran Kishore,Gleiser, Raquel M.,Mohanlall, Viresh,Maharaj, Rajendra,Shashiprabha,Poojary, Vishwanatha
, (2021)
The present study focuses on a series of...
Febuxostat-based amides and some derived heterocycles targeting xanthine oxidase and COX inhibition. Synthesis, in vitro and in vivo biological evaluation, molecular modeling and in silico ADMET studies
Rashad, Aya Y.,Kassab, Shaymaa E.,Daabees, Hoda G.,Abdel Moneim, Ahmed E.,Rostom, Sherif A.F.
, (2021)
Various febuxostat derivatives comprisin...
Pd/Cu-Catalyzed C-H/C-H Cross Coupling of (Hetero)Arenes with Azoles through Arylsulfonium Intermediates
Lin, Zeng-Hui,Tian, Ze-Yu,Zhang, Cheng-Pan
supporting information, p. 4400 - 4405 (2021/06/27)
A highly efficient method for the select...
Synthesis, molecular docking, DFT study of novel N-benzyl-2-(3-cyano-4-isobutoxyphenyl)-4-methylthiazole-5-carboxamide derivatives and their antibacterial activity
Sam Daniel Prabu,Lakshmanan, Sivalingam,Thirumurugan,Ramalakshmi,Arul Antony
, p. 619 - 626 (2020/02/06)
A series of febuxostat based new chemica...
160844-75-7 Process route
-

-
78-77-3
Isobutyl bromide

-
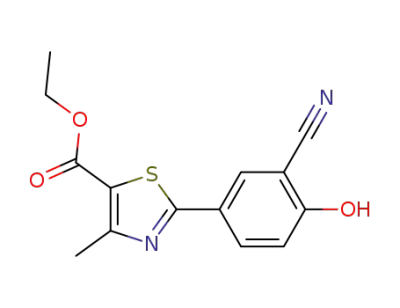
-
161798-02-3
ethyl-2-(3-cyano-4-hydroxy phenyl)-4-methyl thiozole-5-carboxylate

-
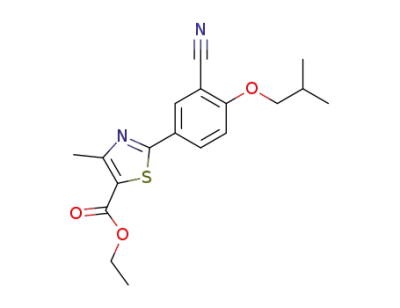
-
160844-75-7
2-(3-cyano-4-isobutyloxyphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester
| Conditions | Yield |
|---|---|
|
With
potassium carbonate; potassium iodide;
In
ethyl acetate;
at 65 - 70 ℃;
for 5h;
Temperature;
Solvent;
Reagent/catalyst;
|
98.2% |
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 75 ℃;
for 8h;
|
92.9% |
|
With
potassium carbonate;
In
1-methyl-pyrrolidin-2-one;
at 90 ℃;
for 3h;
|
88% |
|
With
potassium carbonate;
In
1-methyl-pyrrolidin-2-one;
at 90 ℃;
for 3h;
|
88% |
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 75 ℃;
for 15h;
|
2.28 g |
-

-
78-77-3
Isobutyl bromide

-
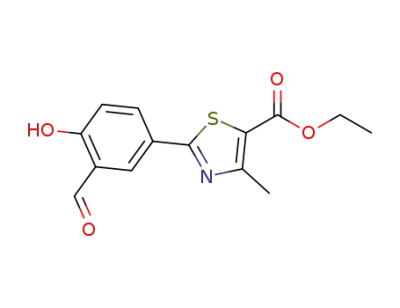
-
161798-01-2
ethyl 2‐(3‐formyl‐4‐hydroxyphenyl)‐4‐methylthiazole‐5‐carboxylate

-

-
160844-75-7
2-(3-cyano-4-isobutyloxyphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester
| Conditions | Yield |
|---|---|
|
ethyl 2‐(3‐formyl‐4‐hydroxyphenyl)‐4‐methylthiazole‐5‐carboxylate;
With
hydroxylamine hydrochloride;
In
dimethyl sulfoxide;
at 40 ℃;
for 0.5h;
With
acetyl chloride;
In
dimethyl sulfoxide;
at 70 - 80 ℃;
Isobutyl bromide;
With
potassium carbonate;
In
dimethyl sulfoxide;
at 20 - 80 ℃;
|
84% |
|
ethyl 2‐(3‐formyl‐4‐hydroxyphenyl)‐4‐methylthiazole‐5‐carboxylate;
With
hydroxylamine hydrochloride;
In
dimethyl sulfoxide;
for 0.5h;
With
acetyl chloride;
In
dimethyl sulfoxide;
at 70 - 80 ℃;
Isobutyl bromide;
With
potassium carbonate;
In
dimethyl sulfoxide;
at 70 - 80 ℃;
for 5h;
|
84% |
160844-75-7 Upstream products
-
609-15-4
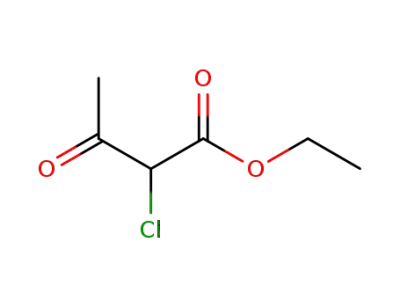
ethyl 2-chloro-3-oxo-butyrate
-
163597-57-7
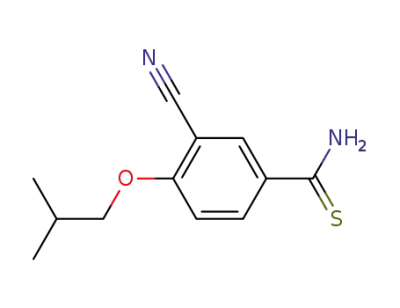
3-cyanoisobutoxybenzothioamide
-
161718-81-6
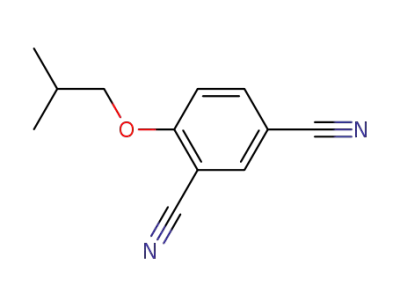
4-isobutoxyisophthalonitrile
-
619-72-7
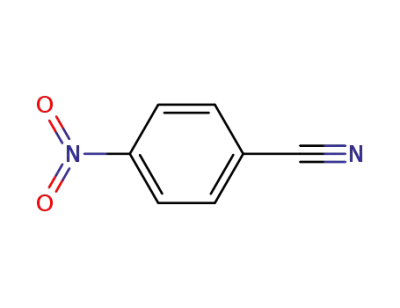
4-nitrobenzonitrile
160844-75-7 Downstream products
-
144060-53-7
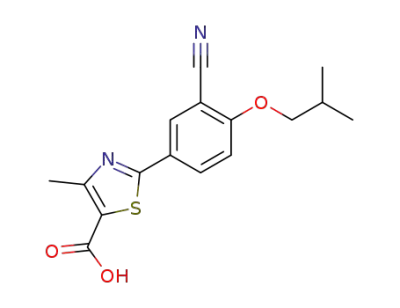
febuxostat
-
1350352-71-4
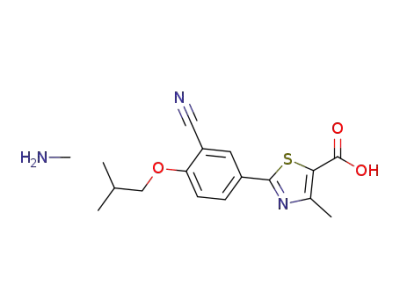
2-[3-cyano-4-(2-methylpropoxy)phenyl]-4-methylthiazole-5-carboxylic acid methylamine
-
1350352-72-5
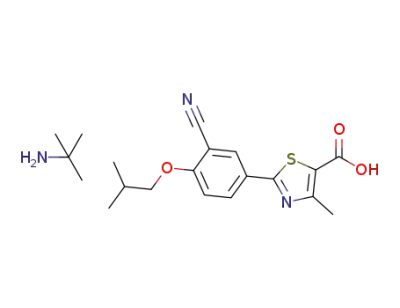
2-[3-cyano-4-(2-methylpropoxy)phenyl]-4-methylthiazole-5-carboxylic acid tert-butylamine
-
1239233-86-3
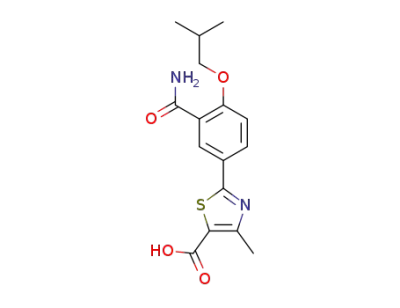
2-[3-carbamoyl-4-(2-methylpropoxy)phenyl]-4-methyl-5-thiazole carboxylic acid


