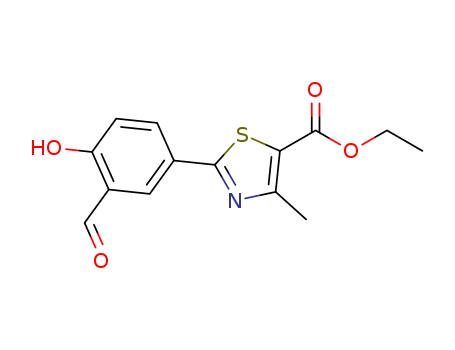
Ethyl 2-(3-Formyl-4-hydroxyphenyl)-4-methylthiazole-5-carboxylate
-
Product Name :
Ethyl 2-(3-Formyl-4-hydroxyphenyl)-4-methylthiazole-5-carboxylate
-
CAS No :
161798-01-2
-
Project State :
Commercial
Application
General Description
Factory Sells Best Quality Ethyl 2-(3-Formyl-4-hydroxyphenyl)-4-methylthiazole-5-carboxylate 161798-01-2 with USP
- Molecular Formula:C14H13NO4S
- Molecular Weight:291.328
- Melting Point:116 °C
- Refractive Index:1.625
- Boiling Point:446.7 °C at 760 mmHg
- PKA:6.81±0.20(Predicted)
- Flash Point:223.9 °C
- PSA:104.73000
- Density:1.335 g/cm3
- LogP:2.81330
ethyl 2-(3-formyl-4-hydroxyphenyl)-4-methyl thiazole-5-carboxylate(Cas 161798-01-2) Usage
InChI:InChI=1/C14H13NO4S/c1-3-19-14(18)12-8(2)15-13(20-12)9-4-5-11(17)10(6-9)7-16/h4-7,17H,3H2,1-2H3
161798-01-2 Relevant articles
Design, synthesis, cytotoxic evaluation and molecular docking studies of novel thiazolyl α-aminophosphonates
Gundluru, Mohan,Badavath, Vishnu Nayak,Shaik, Haroon Yasmin,Sudileti, Murali,Nemallapudi, Bakthavatchala Reddy,Gundala, Sravya,Zyryanov, Grigory V.,Cirandur, Suresh Reddy
, p. 1139 - 1160 (2020/11/16)
Abstract: A new class of thiazolyl α-ami...
Method for synthesizing febuxostat and intermediate thereof
-
Paragraph 0109-0184, (2020/05/02)
The invention relates to a method for sy...
Febuxostat and intermediates and synthesis thereof
-
Paragraph 0081-0156, (2020/05/09)
The invention relates to febuxostat and ...
Synthesis, molecular docking, DFT study of novel N-benzyl-2-(3-cyano-4-isobutoxyphenyl)-4-methylthiazole-5-carboxamide derivatives and their antibacterial activity
Sam Daniel Prabu,Lakshmanan, Sivalingam,Thirumurugan,Ramalakshmi,Arul Antony
, p. 619 - 626 (2020/02/06)
A series of febuxostat based new chemica...
161798-01-2 Process route
-

-
161797-99-5
(2-(4-hydroxyphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester)

-
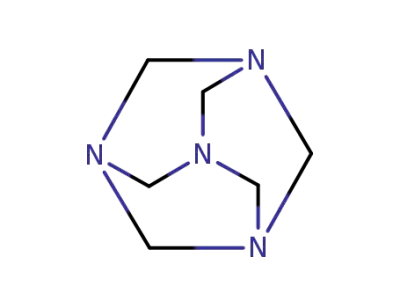
-
100-97-0
hexamethylenetetramine

-

-
161798-01-2
ethyl 2‐(3‐formyl‐4‐hydroxyphenyl)‐4‐methylthiazole‐5‐carboxylate
| Conditions | Yield |
|---|---|
|
With
methanesulfonic acid; boric acid;
In
cyclohexane;
at 75 - 100 ℃;
for 7h;
Temperature;
Reagent/catalyst;
Solvent;
|
91.2% |
|
(2-(4-hydroxyphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester);
With
methanesulfonic acid; boric acid;
In
cyclohexane;
at 80 ℃;
hexamethylenetetramine;
In
cyclohexane;
at 75 ℃;
for 7h;
Temperature;
|
91.2% |
|
With
water; acetic acid; trifluoroacetic acid;
at 100 ℃;
for 2.5h;
|
90% |
|
(2-(4-hydroxyphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester); hexamethylenetetramine;
at 40 - 93 ℃;
for 3h;
Large scale;
With
acetic acid;
In
water;
for 0.333333h;
Temperature;
Reagent/catalyst;
Cooling with ice;
Large scale;
|
81.3% |
|
With
methanesulfonic acid;
at 75 ℃;
for 10h;
|
72.3% |
|
With
trifluoroacetic acid;
for 40h;
Reflux;
|
|
|
(2-(4-hydroxyphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester); hexamethylenetetramine;
With
trifluoroacetic acid;
at 80 ℃;
for 24h;
With
water;
In
toluene;
for 0.166667h;
|
|
|
With
phosphorus pentoxide;
|
|
|
(2-(4-hydroxyphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester); hexamethylenetetramine;
at 75 ℃;
With
acetic acid;
In
water;
at 20 ℃;
|
|
|
With
sulfuric acid;
at 60 - 120 ℃;
|
-

-
161797-99-5
(2-(4-hydroxyphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester)

-
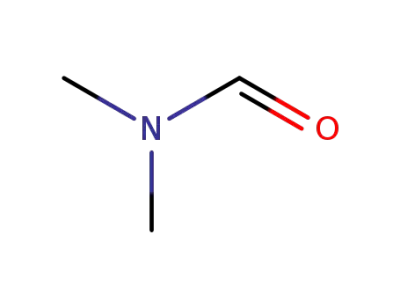
-
68-12-2,33513-42-7
N,N-dimethyl-formamide

-

-
161798-01-2
ethyl 2‐(3‐formyl‐4‐hydroxyphenyl)‐4‐methylthiazole‐5‐carboxylate
| Conditions | Yield |
|---|---|
|
(2-(4-hydroxyphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester);
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -10 ℃;
for 0.2h;
Inert atmosphere;
N,N-dimethyl-formamide;
In
tetrahydrofuran; hexane;
at -10 ℃;
for 0.5h;
Inert atmosphere;
With
acetic acid;
In
tetrahydrofuran; hexane;
at 10 ℃;
for 0.166667h;
Time;
Inert atmosphere;
|
96.6% |
161798-01-2 Upstream products
-
609-15-4
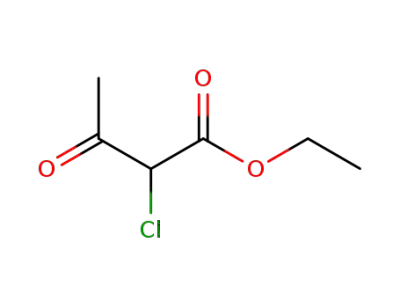
ethyl 2-chloro-3-oxo-butyrate
-
161797-99-5

(2-(4-hydroxyphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester)
-
100-97-0

hexamethylenetetramine
-
25984-63-8
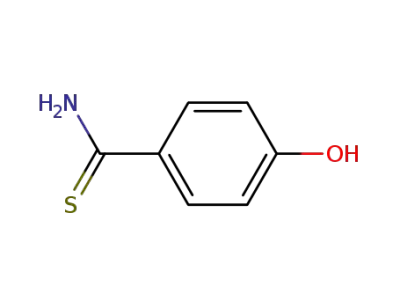
4-hydroxythiobenzamide
161798-01-2 Downstream products
-
161798-03-4
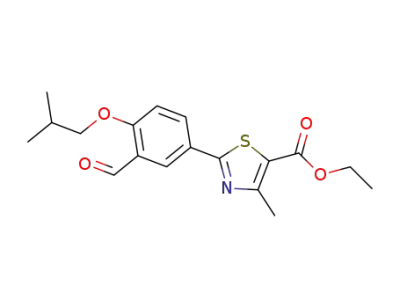
2-(3-formyl-4-isobutyloxyphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester
-
1271738-74-9
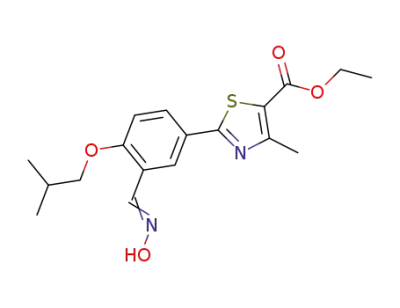
ethyl 2-[3-((hydroxyimino)methyl)-4-(2-methylpropoxy)phenyl]-4-methylthiazole-5-carboxylate
-
144060-53-7
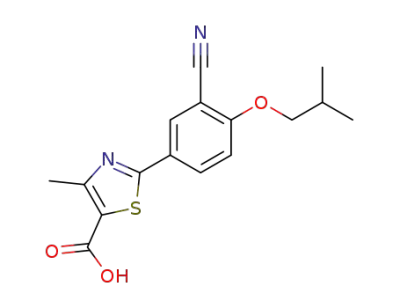
febuxostat
-
160844-75-7

2-(3-cyano-4-isobutyloxyphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester


