N-(9-Fluorenylmethoxycarbonyloxy)succinimide
-
Product Name :
N-(9-Fluorenylmethoxycarbonyloxy)succinimide
-
CAS No :
82911-69-1
-
Project State :
Commercial
Application
General Description
Bulk supply high purity N-(9-Fluorenylmethoxycarbonyloxy)succinimide 82911-69-1, Paid sample available
- Molecular Formula:C19H15NO5
- Molecular Weight:337.332
- Appearance/Colour:White powder
- Vapor Pressure:6.55E-10mmHg at 25°C
- Melting Point:150-153 °C(lit.)
- Refractive Index:1.661
- Boiling Point:494.3 °C at 760 mmHg
- Flash Point:252.7 °C
- PSA:72.91000
- Density:1.42 g/cm3
- LogP:2.95400
N-(9-Fluorenylmethoxycarbonyloxy)succinimide(Cas 82911-69-1) Usage
|
Purification Methods |
Recrystallise the carbonate from CHCl3/Et2O, or from pet ether (b 40-60o). [Pauet Can J Chem 60 976 1982, Lapatsaris et al. Synthesis 671 1983.] |
InChI:InChI=1/C19H15NO5/c21-17-9-10-18(22)20(17)25-19(23)24-11-16-14-7-3-1-5-12(14)13-6-2-4-8-15(13)16/h1-8,16H,9-11H2
82911-69-1 Relevant articles
Method for synthesizing 9-fluorenylmethylsuccinimido carbonate by one-pot two-phase method
-
Paragraph 0011; 0024-0029, (2020/03/03)
The invention relates to the technical f...
Eight-membered ring-containing jadomycins: Implications for non-enzymatic natural products biosynthesis
Robertson, Andrew W.,Martinez-Farina, Camilo F.,Smithen, Deborah A.,Yin, Huimin,Monro, Susan,Thompson, Alison,McFarland, Sherri A.,Syvitski, Raymond T.,Jakeman, David L.
supporting information, p. 3271 - 3275 (2015/03/30)
Jadomycin Oct (1) was isolated from Stre...
The two pathways for effective orthogonal protection of L-ornithine, for amino acylation of 5'-O-Pivaloyl nucleosides, describe the general and important role for the successful imitation, during the synthesis of the model substrates for the ribosomal mimic reaction
Bayryamov, Stanislav G.,Vassilev, Nikolay G.,Petkov, Dimiter D.
experimental part, p. 889 - 898 (2011/12/14)
Bz(NO2)-Orn(Boc)-OCH2CN was synthesized ...
The study of the reaction of terminated oligomerization in the synthesis of oligo-(β1-6)-glucosamines
Gening,Tsvetkov,Pier,Nifantiev
, p. 389 - 399 (2008/02/09)
The applicability of terminated oligomer...
82911-69-1 Process route
-
-
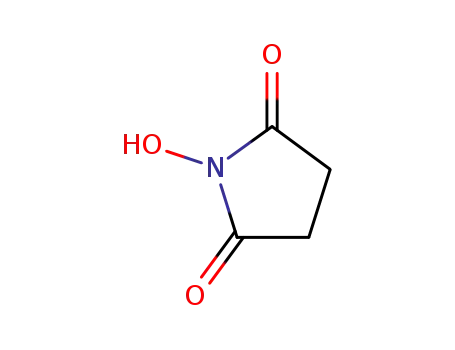
6066-82-6
1-hydroxy-pyrrolidine-2,5-dione

-
-
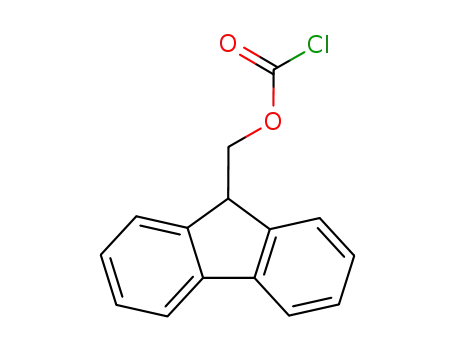
28920-43-6
(fluorenylmethoxy)carbonyl chloride

-
-
82911-69-1
N-(9H-fluoren-2-ylmethoxycarbonyloxy)succinimide
| Conditions | Yield |
|---|---|
|
With
triethylamine;
In
1,4-dioxane;
for 1h;
Ambient temperature;
|
96% |
|
With
sodium carbonate;
In
water; toluene;
at 25 - 30 ℃;
for 2h;
Reagent/catalyst;
Solvent;
|
56.3% |
|
With
sodium carbonate;
In
water; acetone;
for 0.5h;
|
|
|
With
sodium carbonate;
In
water; acetone;
at -10 ℃;
for 1.5h;
|
|
|
With
triethylamine;
In
1,4-dioxane;
|
|
|
With
diisopropylamine; dicyclohexyl-carbodiimide;
In
dichloromethane;
at 0 - 20 ℃;
for 16h;
Inert atmosphere;
|
-
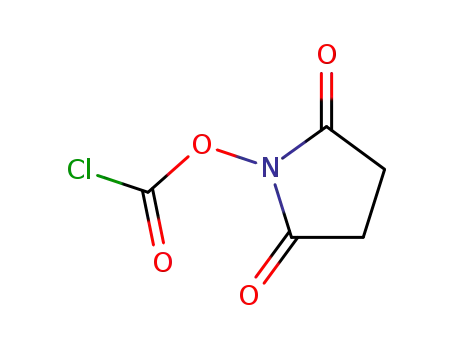
-
15149-73-2
2,5-dioxopyrrolidin-1-yl carbonochloridate

-
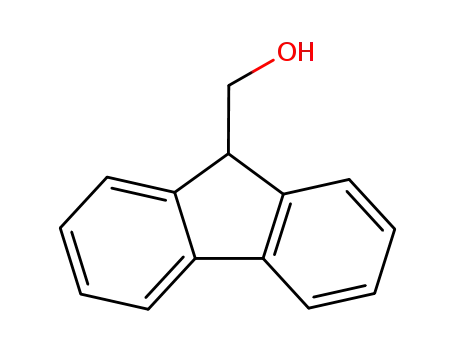
-
24324-17-2
9-Fluorenylmethanol

-

-
82911-69-1
N-(9H-fluoren-2-ylmethoxycarbonyloxy)succinimide
| Conditions | Yield |
|---|---|
|
With
pyridine;
In
dichloromethane;
for 5h;
Ambient temperature;
|
72% |
82911-69-1 Upstream products
-
6066-82-6

1-hydroxy-pyrrolidine-2,5-dione
-
28920-43-6

(fluorenylmethoxy)carbonyl chloride
-
15149-73-2

2,5-dioxopyrrolidin-1-yl carbonochloridate
-
24324-17-2

9-Fluorenylmethanol
82911-69-1 Downstream products
-
73731-37-0
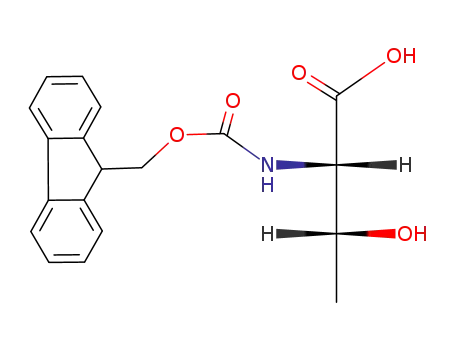
Fmoc-Thr-OH
-
75932-02-4
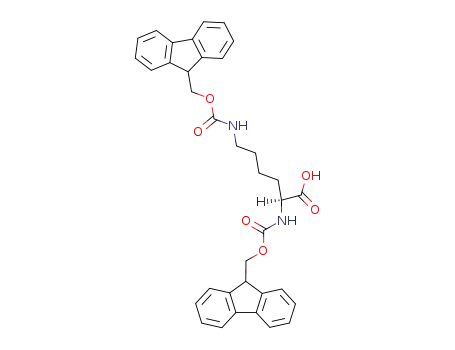
N-2,N-6-bis(9-fluorenylmethyloxycarbonyl)-L-lysine
-
136083-57-3
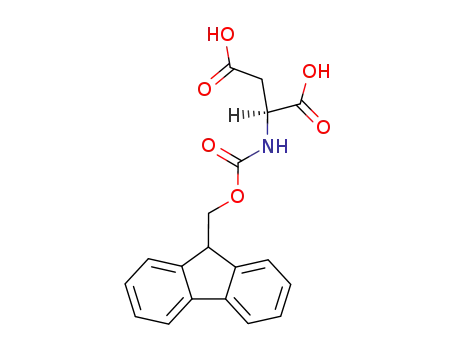
N-α-9-fluorenylmethoxycarbonyl-aspartic acid
-
35661-40-6
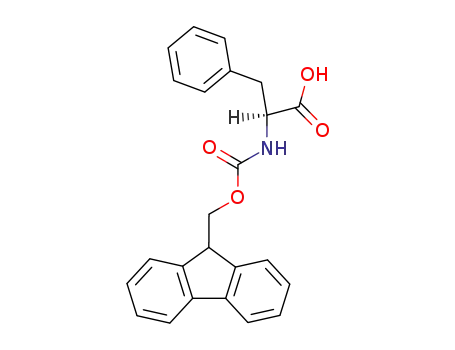
N-Fmoc L-Phe


