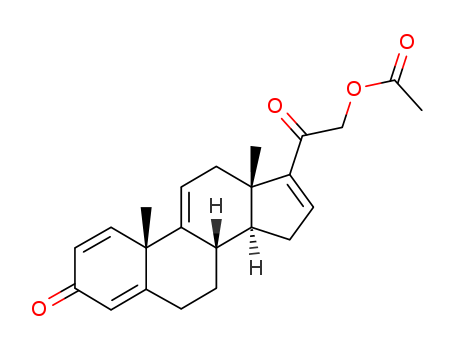
3,20-Dioxopregna-1,4,9(11),16-tetraen-21-yl acetate
-
Product Name :
3,20-Dioxopregna-1,4,9(11),16-tetraen-21-yl acetate
-
CAS No :
37413-91-5
-
Project State :
Commercial
Application
General Description
Cost-effective and customizable 3,20-Dioxopregna-1,4,9(11),16-tetraen-21-yl acetate 37413-91-5 supplier
- Molecular Formula:C23H26O4
- Molecular Weight:366.457
- Vapor Pressure:0mmHg at 25°C
- Melting Point:173-175°C
- Refractive Index:1.585
- Boiling Point:534.568 °C at 760 mmHg
- Flash Point:233.008 °C
- PSA:60.44000
- Density:1.211 g/cm3
- LogP:3.88290
3,20-Dioxopregna-1,4,9(11),16-tetraen-21-yl acetate(Cas 37413-91-5) Usage
InChI:InChI=1/C23H26O4/c1-14(24)27-13-21(26)20-7-6-18-17-5-4-15-12-16(25)8-10-22(15,2)19(17)9-11-23(18,20)3/h7-10,12,17-18H,4-6,11,13H2,1-3H3/t17-,18-,22-,23-/m0/s1
37413-91-5 Relevant articles
Optimization of the synthesis of a key intermediate for the preparation of glucocorticoids
Jouve, Romain,Thery, Vincent,Ducki, Sylvie,Helfenbein, Julie,Thiery, Jean-Christophe,Job, Aurélie,Picard, Elodie,Mallet, Christophe,Ripoche, Isabelle,Bennis, Khalil
, p. 14 - 21 (2018)
A short and efficient synthesis, based o...
An efficient procedure for the synthesis of 21-acetoxypregna-1,4,9(11),16-tetraene-3,20-dione
Huy, Luu D.,Diep, Nguyen T.,Vu, Tran K.,Savinova, Tatiana S.,Donova, Marina V.
, p. 225 - 231 (2020/04/27)
Background: Halogenated corticosteroids ...
Preparation method of tetraene acetate
-
Paragraph 0066-0086, (2019/12/25)
The invention discloses a preparation me...
Method for preparing tetraene acetate
-
Paragraph 0021-0023; 0026; 0029; 0032; 0035; 0038, (2019/01/07)
The invention provides a method for prep...
37413-91-5 Process route
-
-
4380-55-6
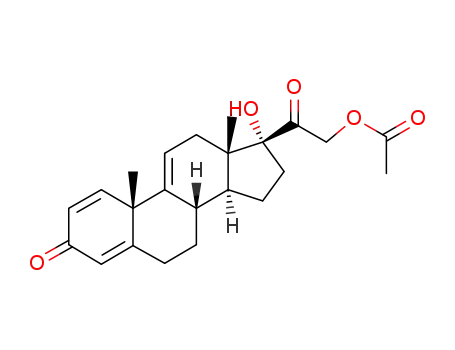
pregna-1,4,9(11)-triene-17α,21-diol-3,20-dione 21-acetate

-
![2-((10S,13S,14S)-10,13-dimethyl-3-oxo-6,7,8,10,12,13,14,15-octahydro-3H-cyclopenta[a]phenanthren-17-yl)-2-oxoethyl acetate](/upload/2025/9/bb44d903-af99-4b55-80b5-c8edf4abe37d.png)
-
37413-91-5
2-((10S,13S,14S)-10,13-dimethyl-3-oxo-6,7,8,10,12,13,14,15-octahydro-3H-cyclopenta[a]phenanthren-17-yl)-2-oxoethyl acetate
| Conditions | Yield |
|---|---|
|
pregna-1,4,9(11)-triene-17α,21-diol-3,20-dione 21-acetate;
With
pyridine; N-chloro-succinimide;
at -15 ℃;
for 1h;
Inert atmosphere;
With
sulfur dioxide;
at -10 ℃;
for 0.5h;
Inert atmosphere;
|
98.8% |
-
-
52-21-1
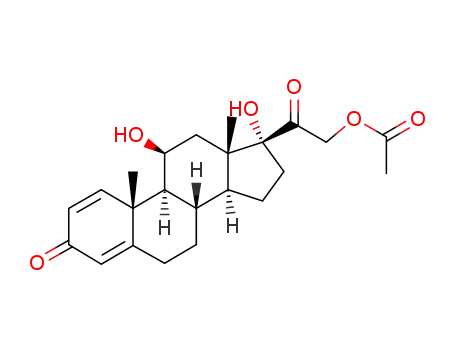
prednisolone 21-acetate

-
![2-((10S,13S,14S)-10,13-dimethyl-3-oxo-6,7,8,10,12,13,14,15-octahydro-3H-cyclopenta[a]phenanthren-17-yl)-2-oxoethyl acetate](/upload/2025/9/bb44d903-af99-4b55-80b5-c8edf4abe37d.png)
-
37413-91-5
2-((10S,13S,14S)-10,13-dimethyl-3-oxo-6,7,8,10,12,13,14,15-octahydro-3H-cyclopenta[a]phenanthren-17-yl)-2-oxoethyl acetate
| Conditions | Yield |
|---|---|
|
prednisolone 21-acetate;
With
pyridine; N-chloro-succinimide;
at -15 - 20 ℃;
for 0.416667h;
Inert atmosphere;
With
sulfur dioxide;
for 1h;
Inert atmosphere;
|
83.5% |
37413-91-5 Upstream products
-
1035-69-4
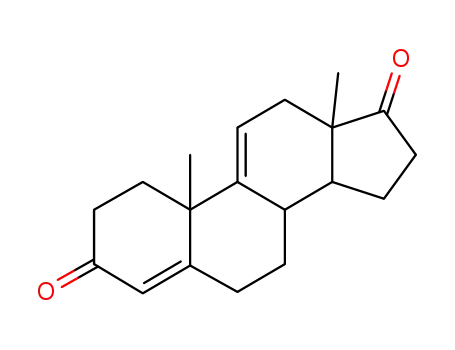
Androstadien-(4,9,(11))-dion-3,17
-
23460-76-6
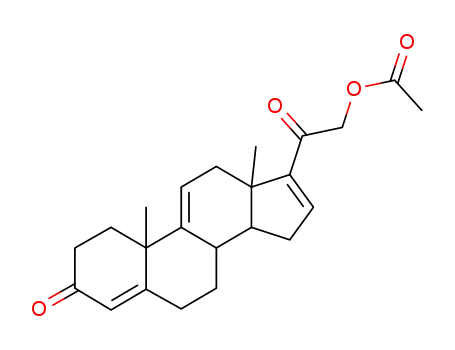
21-Acetoxypregna-4,9(11),16-trien-3,20-dion
-
23460-76-6
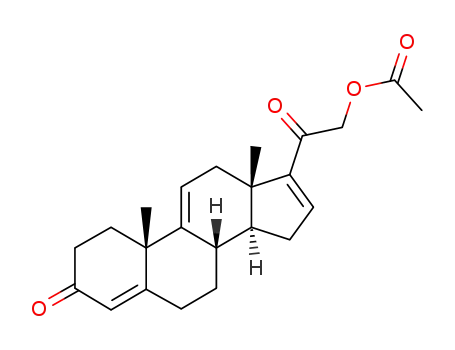
21-acetoxy-pregna-4,9(11),16-triene-3,20-dinone
-
108-75-8
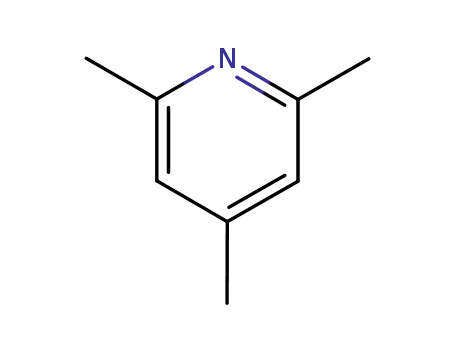
2,4,6-trimethyl-pyridine
37413-91-5 Downstream products
-
77017-20-0
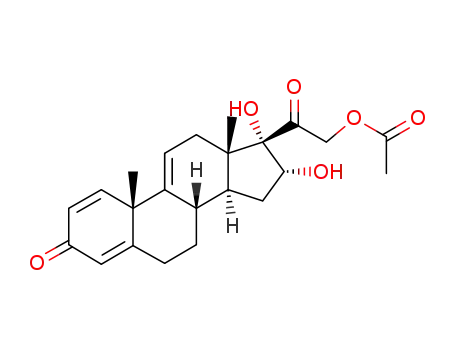
16α,17α,21-trihydroxypregna-1,4,9(11)-triene-3,20-dione-21-acetate
-
10106-41-9
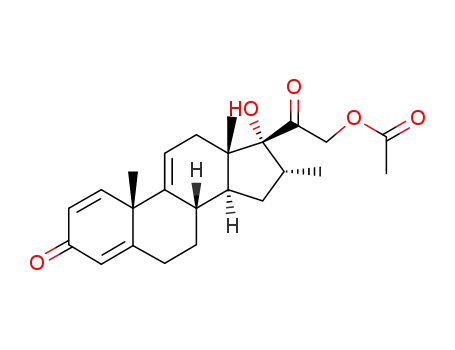
17α,21-dihydroxy-16α-methyl-1,4,9(11)-pregnatriene-3,20-dione 21-acetate
-
4258-83-7
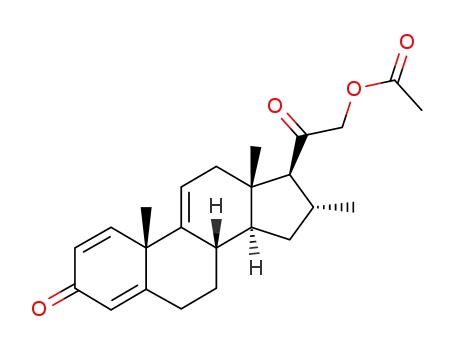
2-oxo-2-((8S,10S,13S,14S,16R,17S)-10,13,16-trimethyl-3-oxo-6,7,8,10,12,13,14,15,16,17-decahydro-3H-cyclopenta[a]phenanthren-17-yl)ethyl acetate
-
2476-74-6
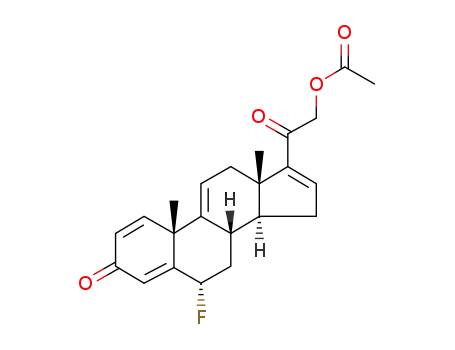
6α-fluoro-1,4,9,16-tetraenepregna-3,20-dione-21-acetate


