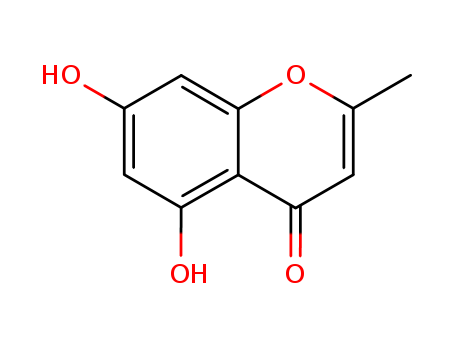
2-Methyl-5,7-dihydroxychromone
-
Product Name :
2-Methyl-5,7-dihydroxychromone
-
CAS No :
1013-69-0
-
Project State :
Commercial
Application
General Description
Buy cost-effective 99% pure 2-Methyl-5,7-dihydroxychromone 1013-69-0 now
- Molecular Formula:C10H8O4
- Molecular Weight:192.171
- Vapor Pressure:8.59E-07mmHg at 25°C
- Melting Point:279 °C
- Refractive Index:1.651
- Boiling Point:394.6 °C at 760 mmHg
- PKA:6.58±0.40(Predicted)
- Flash Point:164 °C
- PSA:70.67000
- Density:1.456 g/cm3
- LogP:1.51260
2-Methyl-5,7-dihydroxychromone(Cas 1013-69-0) Usage
|
General Description |
2-Methyl-5,7-dihydroxychromone is a chemical compound that belongs to the family of flavones, which are commonly found naturally in various plant species. These are yellow pigments that provide coloration for some flowers, fruits, and leaves. The specific compound, 2-methyl-5,7-dihydroxychromone, exhibits antioxidant properties, which means it can help protect cells from damages caused by harmful molecules known as free radicals. Studies have also suggested that flavones like this one might have potential health benefits, including anti-inflammation, anti-allergy, and anti-cancer effects, although more research is needed in this area. |
InChI:InChI=1/C10H8O4/c1-5-2-7(12)10-8(13)3-6(11)4-9(10)14-5/h2-4,11,13H,1H3
1013-69-0 Relevant articles
BIFLORIN, A CHROMONE-C-GLUCOSIDE FROM PANCRATIUM BIFLORUM
Ghosal, Shibnath,Kumar, Yatendra,Singh, Shripati, Ahad, Kamal
, p. 2591 - 2594 (1983)
A new polyoxigenated chromone-C-glucosid...
A plant type III polyketide synthase that produces pentaketide chromone
Abe, Ikuro,Utsumi, Yoriko,Oguro, Satoshi,Morita, Hiroyuki,Sano, Yukie,Noguchi, Hiroshi
, p. 1362 - 1363 (2005)
A novel plant-specific type III polyketi...
Engineered biosynthesis of plant polyketides: Manipulation of chalcone synthase
Abe, Ikuro,Watanabe, Tatsuya,Morita, Hiroyuki,Kohno, Toshiyuki,Noguchi, Hiroshi
, p. 499 - 502 (2006)
Chalcone synthase (CHS) is a plant-speci...
-
Schmid,Meijer
, p. 748,751 (1948)
-
Engineered biosynthesis of plant polyketides: Chain length control in an octaketide-producing plant type III polyketide synthase
Abe, Ikuro,Oguro, Satoshi,Utsumi, Yoriko,Sano, Yukie,Noguchi, Hiroshi
, p. 12709 - 12716 (2005)
The chalcone synthase (CHS) superfamily ...
Studies on medical resources. XXXI. Component of the leaves of Staphylea bumalada DC
Morita,Shimizu,Uchida
, p. 1311 - 1312 (1968)
-
TMSI-Promoted vinylogous michael addition of siloxyfuran to 2-substituted chromones: A general approach for the total synthesis of chromanone lactone natural products
Liu, Jie,Li, Zhanchao,Tong, Pei,Xie, Zhixiang,Zhang, Yuan,Li, Ying
, p. 1632 - 1643 (2015)
A concise and facile synthetic protocol ...
Anti-inflammatory chromone alkaloids and glycoside from Dysoxylum binectariferum
Kumar, Vikas,Gupta, Mehak,Gandhi, Sumit G.,Bharate, Sonali S.,Kumar, Ajay,Vishwakarma, Ram A.,Bharate, Sandip B.
, p. 3974 - 3978 (2017)
Herein we report isolation of a new chro...
Structure-based engineering of a plant type III polyketide synthase: Formation of an unnatural nonaketide naphthopyrone
Abe, Ikuro,Morita, Hiroyuki,Oguro, Satoshi,Noma, Hisashi,Wanibuchi, Kiyofumi,Kawahara, Nobuo,Goda, Yukihiro,Noguchi, Hiroshi,Kohno, Toshiyuki
, p. 5976 - 5980 (2007)
Pentaketide chromone synthase (PCS) from...
CHROMONE GLYCOSIDES DROM SCHUMANNIOPHYTON MAGNIFICUM
Tane, Pierre,Ayafor, Johnson F.,Sondengam, B. Luc,Connolly, Joseph D.
, p. 1004 - 1007 (1990)
Two new chromone glycosides, schumanniof...
-
Meijer,Schmid
, p. 1603,1606 (1948)
-
Flavonoid-based inhibitors of the Phi-class glutathione transferase from black-grass to combat multiple herbicide resistance
Brazier-Hicks, Melissa,Coxon, Christopher R.,Cummins, Ian,Edwards, Robert,Eno, Rebecca F. M.,Freitag-Pohl, Stefanie,Hughes, David J.,Mitchell, Glynn,Moore, Jenny,Onkokesung, Nawaporn,Pohl, Ehmke,Schwarz, Maria,Steel, Patrick G.,Straker, Hannah E.,Wortley, David J.
supporting information, p. 9211 - 9222 (2021/11/16)
The evolution and growth of multiple-her...
Synthesis and Antifungal Activity of Chromones and Benzoxepines from the Leaves of Ptaeroxylon obliquum
Malefo, Modibo S.,Ramadwa, Thanyani E.,Famuyide, Ibukun M.,McGaw, Lyndy J.,Eloff, Jacobus N.,Sonopo, Molahlehi S.,Selepe, Mamoalosi A.
, p. 2508 - 2517 (2020/09/15)
This study reports the first total synth...
Derivatives of Natural Product Agrimophol as Disruptors of Intrabacterial pH Homeostasis in Mycobacterium tuberculosis
Wu, Jie,Mu, Ran,Sun, Mingna,Zhao, Nan,Pan, Miaomiao,Li, Hongshuang,Dong, Yi,Sun, Zhaogang,Bai, Jie,Hu, Minwan,Nathan, Carl F.,Javid, Babak,Liu, Gang
, p. 1087 - 1104 (2019/05/22)
This article reports the rational medici...
Novel diphenylmethyl compounds having mycobacterium tuberculosis inhibitory activity
-
Paragraph 0845; 0846; 0849; 0850, (2019/02/13)
The invention relates to novel diphenylm...
1013-69-0 Process route
-
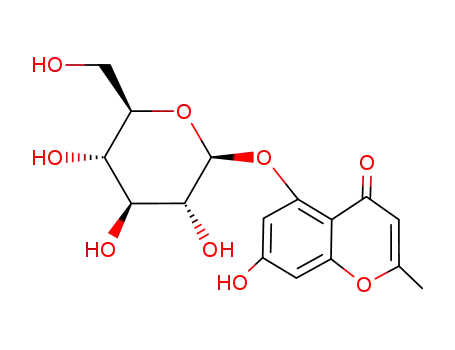
-
128396-15-6
noreugenin 7-O-β-D-glucoside

-
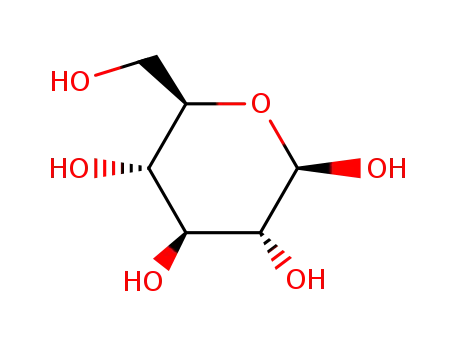
-
492-61-5
β-D-glucose

-
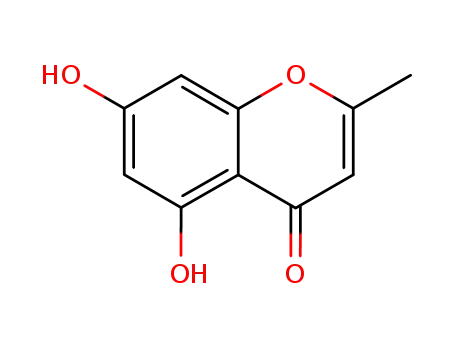
-
1013-69-0
eugenin
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; water;
for 3h;
Reflux;
|
-
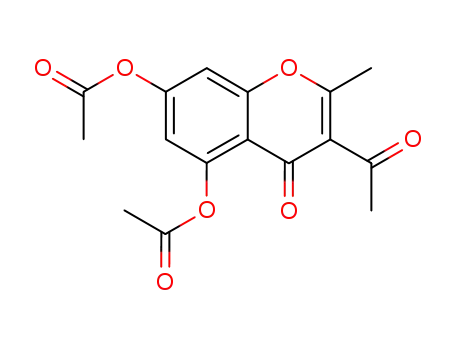
-
1162-81-8
3-Acetyl-5,7-bis(acetyloxy)-2-methyl-4H-1-benzopyran-4-one

-

-
1013-69-0
eugenin
| Conditions | Yield |
|---|---|
|
With
sodium carbonate;
for 2h;
Reagent/catalyst;
Reflux;
|
93.6% |
|
With
sodium carbonate;
for 1h;
Heating;
|
75% |
|
With
hydrogenchloride; sodium carbonate;
Yield given. Multistep reaction;
1.) reflux, 1 h;
|
|
|
Multi-step reaction with 2 steps
1: aqueous hydrochloric acid
2: aqueous sodium carbonate-solution
With
hydrogenchloride; sodium carbonate;
|
|
|
With
sodium carbonate;
In
water;
for 1h;
Heating / reflux;
|
1013-69-0 Upstream products
-
480-34-2
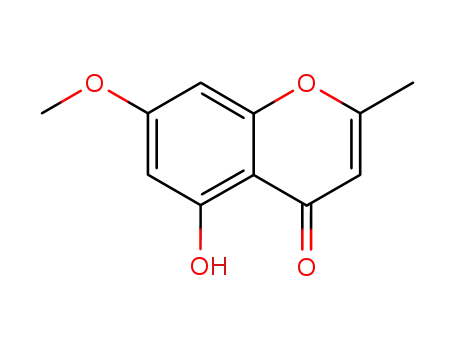
eugenin
-
480-27-3
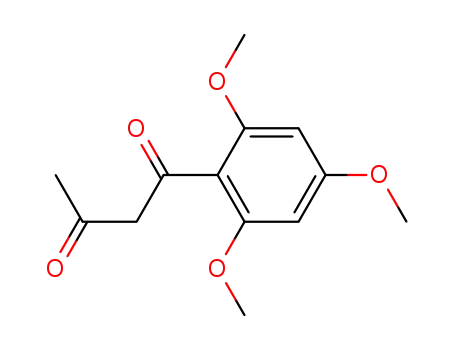
1-Methyl-3-(2',4',6'-trimethoxyphenyl)propan-1,3-dione
-
1022-78-2
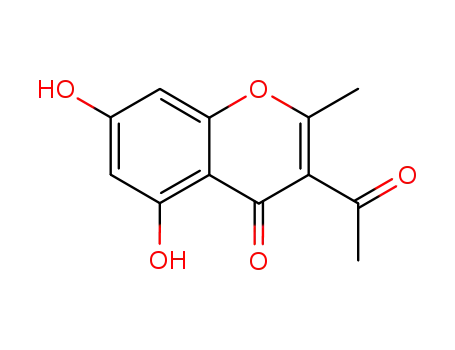
3-acetyl-5,7-dihydroxy-2-methyl-chromen-4-one
-
480-66-0
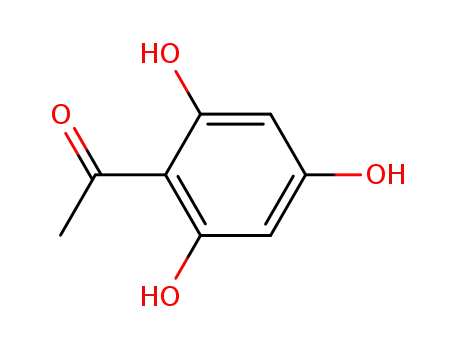
2,4,6-trihydroxyacetophenone
1013-69-0 Downstream products
-
101452-07-7
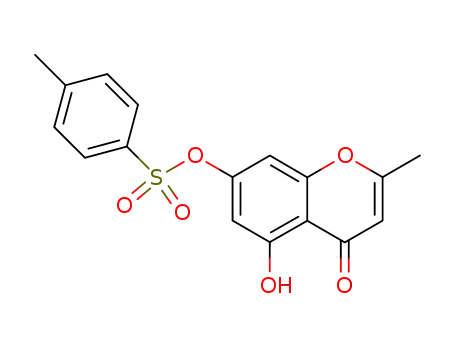
5-hydroxy-2-methyl-7-(toluene-4-sulfonyloxy)-chromen-4-one
-
102664-19-7
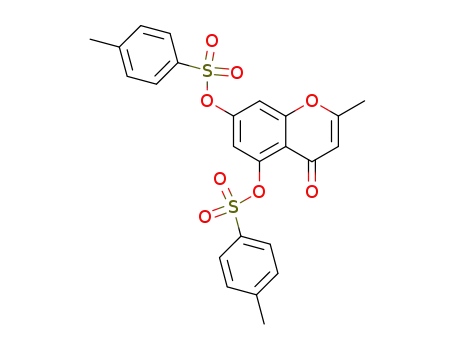
2-methyl-5,7-bis-(toluene-4-sulfonyloxy)-chromen-4-one
-
480-34-2

eugenin
-
480-12-6
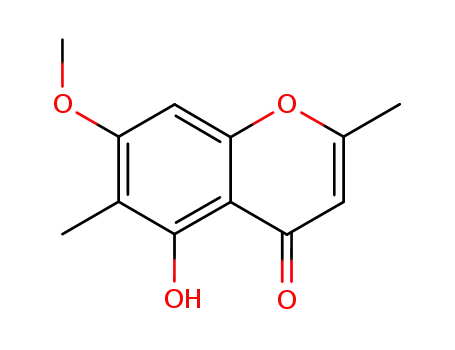
eugenitin


