Dutasteride
-
Product Name :
Dutasteride
-
CAS No :
164656-23-9
-
Project State :
Commercial
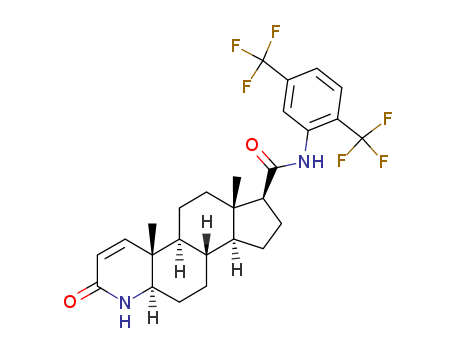
Application
General Description
Good factory supply good Dutasteride 164656-23-9
- Molecular Formula:C27H30F6N2O2
- Molecular Weight:528.538
- Appearance/Colour:white crystalline solid
- Vapor Pressure:0mmHg at 25°C
- Melting Point:242-250 °C
- Refractive Index:1.523
- Boiling Point:620.3 °C at 760 mmHg
- PKA:13.32±0.70(Predicted)
- Flash Point:329 °C
- PSA:58.20000
- Density:1.303 g/cm3
- LogP:6.97790
Dutasteride(Cas 164656-23-9) Usage
|
Biological Functions |
Similar to finasteride, dutasteride is a competitive and mechanism-based inhibitor not only of type 2 but also of type 1 5α-reductase isoenzymes, with which stable enzyme-NADP adduct complexes are formed, inhibiting the conversion of testosterone to DHT. The suppression of both type 1 and type 2 isoforms results in greater and more consistent reduction of plasma DHT than that observed for finasteride. The more effective dual inhibition of type 1 and type 2 5α-reductase isoforms lowers circulating DHT to a greater extent than with finasteride and shows advantages in treating BPH and other disease states (e.g., prostate cancer) that are DHT-dependent. |
|
Biochem/physiol Actions |
Dutasteride is a potent dual inhibitor of 5α-reductase isoenzymes types 1 and 2 (IC50 = 6 nM 5-AR1; 7 nM 5-AR2). Dutasteride blocks testosterone conversion to dihydrotesterone, and is used clinically for treating benign prostatic hyperplasia (BPH). |
|
Pharmacokinetics |
The maximum effect of 0.5 mg daily doses of dutasteride on the suppression of DHT is dose-dependent and is observed within 1 to 2 weeks. After 2 weeks of 0.5 mg daily dosing, median plasma DHT concentrations were reduced by 90%, and after 1 year, the median decrease in plasma DHT was 94%. The median increase in plasma testosterone was 19% but remained within the physiological range. The drug also reduced serum prostatic specific antigen by approximately 50% at 6 months and total prostate volume by 25% at 2 years. Dutasteride produced improvements in quality of life and peak urinary flow rate and reduction of acute urinary retention without the need for surgery. |
|
Side effects |
The main side effects are ED, decreased libido, gynecomastia, and ejaculation disorders. Long-term use (>4 years), however, did not reveal increased onset of sexual side effects. In addition, the combination of dutasteride and tamsulosin is well-tolerated and has the added advantage of rapid symptomatic relief. |
|
Synthesis |
Dutasteride can be prepared from 3-oxo-4-androstene-17β-carboxylic acid by several ways in 6 or 8 steps. In the preparation of dutasteride, the introduction of the carbon-carbon double bond in conjugation with C-3 carbonyl carbon of azaandrosteriods is one of the most important chemical reaction.an efficient synthesis of dutasteride: utilizing benzoyl group as novel lactamic protecting group |
|
in vivo |
dutasteride, which inhibits both 5αr1/5αr2, is efficacious in blocking prostate cancer development or progression in c57bl/6 tramp x fvb mice [2]. |
|
Drug interactions |
Potentially hazardous interactions with other drugsNone known |
|
Metabolism |
Dutasteride is metabolised by the cytochrome P450 isoenzymes CYP3A4 and CYP3A5, and most of a dose is excreted as metabolites in the faeces. |
|
Mode of action |
The human body contains type I and type II 5α reductase, with type II found mainly in the prostate, and type I found mainly in the liver and skin. 5α reductase is the main cause for continuous benign prostate enlargement; it promotes the transformation of testosterone in patients’ prostate into the more active dihydrotestosterone, thus causing prostate cells to enlarge and the prostate to swell. Dutasteride can inhibit both type I and II 5α reductase at the same time. This type of simultaneous inhibiting mechanism can rapidly and continuously reduce prostate size, dramatically improve urination, and reduce the risk fo acute urinary retention and its related prostate surgeries. |
|
Clinical claims and research |
The American FDA approved a 2-year multicenter randomized double-blind control clinical trial – the first long term clinical assessment of the combined usage of Dutasteride and α receptor blockers. Included subjects were male patients with moderate to severe prostate enlargement (ages greater than or equal to 50, prostate volume (PV) ≥30 cc, serum prostate specific antigen (PSA) levels 1.5-10ng/ml, 5ml/sec < maximum urinary flow (Qmax) ≤15ml/sec, minimum urination ≥ 125ml, international prostate symptom score (IPSS) ≥ 12). Patients were first given a placebo for 4 weeks and then were randomly given either 0.5mg/day of Dutasteride and 0.4mg/day of Tamsulosin, only 0.5mg/day of Dutasteride, or only 0.4mg/day of Tamsulosin.Results showed: After 12-24 months, the combined usage of Dutasteride with Tamsulosin had better curative effects than did individual usage. |
|
references |
[1] schmidt lj1, murillo h, tindall dj. gene expression in prostate cancer cells treated with the dual 5 alpha-reductase inhibitor dutasteride. j androl. 2004 nov-dec;25(6):944-53.[2] opoku-acheampong ab1, unis d, henningson jn, beck ap, lindshield bl.preventive and therapeutic efficacy of finasteride and dutasteride in tramp mice. plos one. 2013 oct 18;8(10):e77738. doi: 10.1371/journal.pone.0077738. ecollection 2013. |
|
Definition |
ChEBI: Dutasteride is an aza-steroid that is inasteride in which the tert-butyl group is replaced by a 2,5-bis(trifluoromethyl)phenyl group. A synthetic 4-azasteroid, dutasteride is a selective inhibitor of both the type 1 and type 2 isoforms of steroid 5alpha-reductase, an intracellular enzyme that converts testosterone to 5alpha-dihydrotestosterone. Dutasteride is used for the treatment of symptomatic benign prostatic hyperplasia in men with an enlarged prostate gland. It has a role as an EC 1.3.1.22 [3-oxo-5alpha-steroid 4-dehydrogenase (NADP(+))] inhibitor and an antihyperplasia drug. It is an aza-steroid, a member of (trifluoromethyl)benzenes and a delta-lactam. It derives from a hydride of a 5alpha-androstane. |
|
Brand name |
Avodart (GlaxoSmithKline). |
InChI:InChI=1/C27H30F6N2O2/c1-24-11-9-17-15(4-8-21-25(17,2)12-10-22(36)35-21)16(24)6-7-19(24)23(37)34-20-13-14(26(28,29)30)3-5-18(20)27(31,32)33/h3,5,10,12-13,15-17,19,21H,4,6-9,11H2,1-2H3,(H,34,37)(H,35,36)/t15?,16?,17?,19-,21?,24+,25-/m1/s1
164656-23-9 Relevant articles
A method of preparation he male amine
-
Paragraph 0012-0013, (2018/09/26)
The invention belongs to the field of ph...
Production process of high-purity dutasteride
-
Paragraph 0046; 0047; 0053-0055; 0058-0060; 0069-0077, (2018/04/02)
The invention discloses a purification p...
A androst - 17β - N - (2, 5 - (trifluoromethyl)-) IBZM preparation method
-
Paragraph 0039; 0040; 0041; 0046; 0047, (2018/01/02)
The invention provides a preparation met...
Preparation method of dutasteride
-
Paragraph 0017; 0028; 0029; 0032, (2017/06/29)
The invention relates to a preparation m...
164656-23-9 Process route
-
-
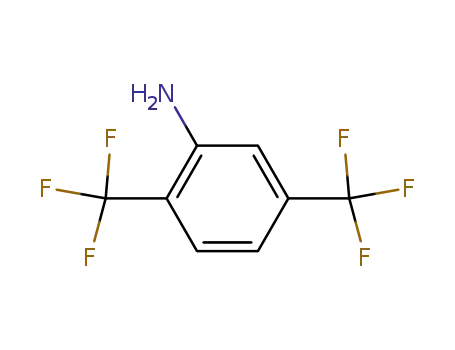
328-93-8
2,5-bis(trifluoromethyl)aniline

-
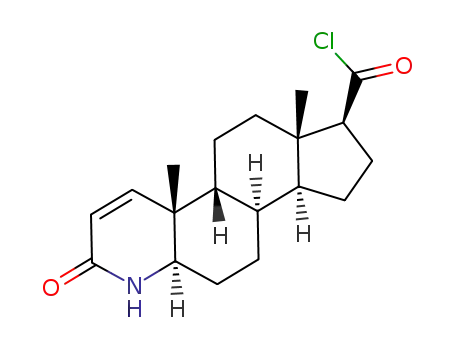
-
(S)-3-keto-4-aza-5α-androstane-1-ene-17β-formyl chloride

-
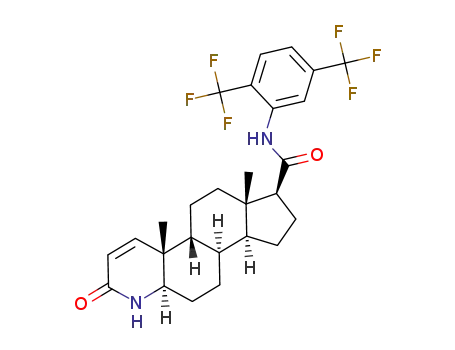
-
1796930-46-5,164656-23-9
(5α,17β)-N-(2,5-bis(trifluoromethyl)phenyl)-3-oxo-4-azaandrost-1-ene-17-carboxamide
| Conditions | Yield |
|---|---|
|
With
pyridine;
In
toluene;
at 90 ℃;
for 3h;
Temperature;
|
95.2% |
-

-
328-93-8
2,5-bis(trifluoromethyl)aniline

-
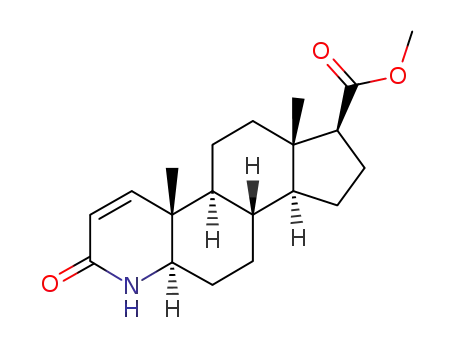
-
103335-41-7
methyl 3-oxo-4-aza-5α-androst-1-ene-17β-carboxylate

-
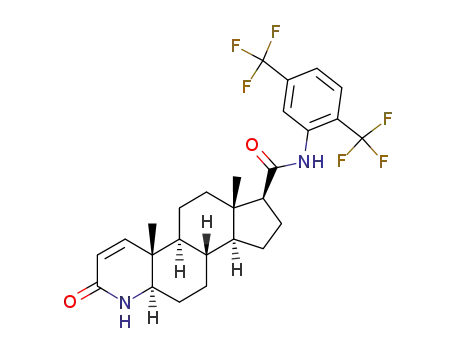
-
1796930-46-5,164656-23-9
dutasteride
| Conditions | Yield |
|---|---|
|
With
1,8-diazabicyclo[5.4.0]undec-7-ene;
In
2-methyltetrahydrofuran;
at 80 ℃;
for 12h;
|
78.23% |
164656-23-9 Upstream products
-
328-92-7
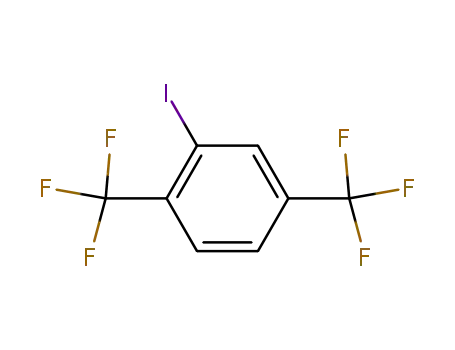
2-iodo-1,4-bis-(trifluoromethyl)-benzene
-
104214-61-1
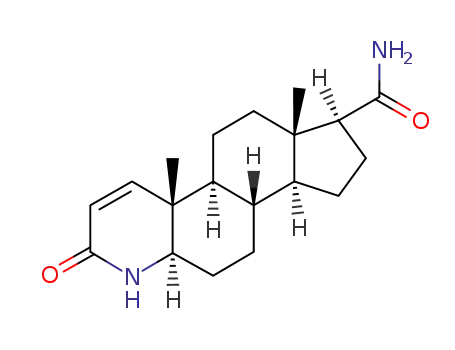
3-Oxo-4-aza-5alpha-androst-1-ene-17beta-carboxamide
-
1260388-53-1
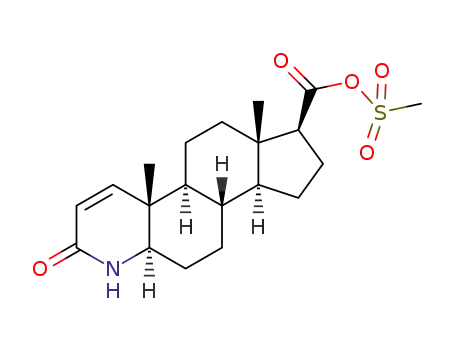
C20H29NO5S
-
328-93-8

2,5-bis(trifluoromethyl)aniline


