Azilsartan medoxomil potassium
-
Product Name :
Azilsartan medoxomil potassium
-
CAS No :
1417576-00-1
-
Project State :
Commercial
Application
General Description
Cost-effective and customizable Azilsartan medoxomil potassium 1417576-00-1 in stock
- Molecular Formula:C28H20N4O8
- Molecular Weight:540.489
1417576-00-1 Relevant articles
Liquid chromatography/tandem mass spectrometry study of forced degradation of azilsartan medoxomil potassium
Swain, Debasish,Patel, Prinesh N.,Palaniappan, Ilayaraja,Sahu, Gayatri,Samanthula, Gananadhamu
, p. 1437 - 1447 (2015/07/15)
Rationale Azilsartan medoxomil potassium...
Improved process for azilsartan medoxomil: A new angiotensin receptor blocker
Radl, Stanislav,Cerny, Josef,Stach, Jan,Gablikova, Zuzana
, p. 77 - 86 (2013/03/28)
An improved process for the active pharm...
1417576-00-1 Process route
-
-
863031-21-4
Azilsartan medoxomil

-
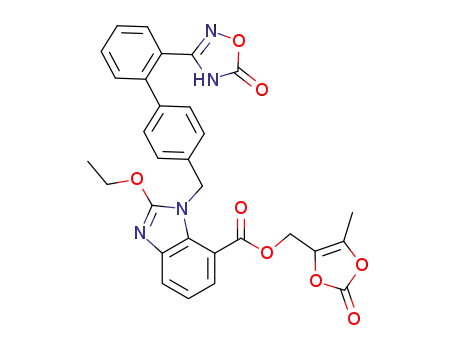
![(5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 2-oxo-3-((2’-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)-[1,1’-biphenyl]-4-yl)methyl)-2,3-dihydro-1H-benzo[d]imidazole-4-carboxylate](/upload/2025/9/37fd8e25-cf84-4c05-95f3-1cb9599ed64b.png)
-
1417576-00-1
(5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 2-oxo-3-((2’-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)-[1,1’-biphenyl]-4-yl)methyl)-2,3-dihydro-1H-benzo[d]imidazole-4-carboxylate
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
water; acetone;
for 1h;
Reflux;
|
73% |
-
![methyl 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxy-benzimidazole-7-carboxylate](/upload/2025/9/0e15a5f1-f10c-4dc3-bbb4-d54f3c51ffa4.png)
-
139481-44-0
methyl 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxy-benzimidazole-7-carboxylate

-
![(5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 2-oxo-3-((2’-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)-[1,1’-biphenyl]-4-yl)methyl)-2,3-dihydro-1H-benzo[d]imidazole-4-carboxylate](/upload/2025/9/37fd8e25-cf84-4c05-95f3-1cb9599ed64b.png)
-
1417576-00-1
(5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 2-oxo-3-((2’-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)-[1,1’-biphenyl]-4-yl)methyl)-2,3-dihydro-1H-benzo[d]imidazole-4-carboxylate
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 5 steps
1: hydroxylamine hydrochloride / dimethyl sulfoxide; water / 18 h / 90 °C
2: potassium carbonate / dimethyl sulfoxide / 4 h / 20 °C
3: sodium hydroxide; water / 3 h / 50 °C
4: potassium carbonate; p-toluenesulfonyl chloride; dmap / N,N-dimethyl acetamide / 3 h / 30 °C
5: hydrogenchloride / acetone; water / 1 h / Reflux
With
hydrogenchloride; dmap; hydroxylamine hydrochloride; water; potassium carbonate; p-toluenesulfonyl chloride; sodium hydroxide;
In
N,N-dimethyl acetamide; water; dimethyl sulfoxide; acetone;
|
|
|
Multi-step reaction with 5 steps
1: hydroxylamine hydrochloride / dimethyl sulfoxide; water / 18 h / 90 °C
2: 1,8-diazabicyclo[5.4.0]undec-7-ene / dimethyl sulfoxide / 4 h / 20 °C
3: sodium hydroxide; water / 3 h / 50 °C
4: potassium carbonate; p-toluenesulfonyl chloride; dmap / N,N-dimethyl acetamide / 3 h / 30 °C
5: hydrogenchloride / acetone; water / 1 h / Reflux
With
hydrogenchloride; dmap; hydroxylamine hydrochloride; water; potassium carbonate; 1,8-diazabicyclo[5.4.0]undec-7-ene; p-toluenesulfonyl chloride; sodium hydroxide;
In
N,N-dimethyl acetamide; water; dimethyl sulfoxide; acetone;
|
|
|
Multi-step reaction with 5 steps
1: hydroxylamine hydrochloride / dimethyl sulfoxide; water / 18 h / 90 °C
2: sodium methylate / dimethyl sulfoxide; methanol / 0.33 h / 10 - 21 °C
3: sodium hydroxide; water / 3 h / 50 °C
4: potassium carbonate; p-toluenesulfonyl chloride; dmap / N,N-dimethyl acetamide / 3 h / 30 °C
5: hydrogenchloride / acetone; water / 1 h / Reflux
With
hydrogenchloride; dmap; hydroxylamine hydrochloride; water; sodium methylate; potassium carbonate; p-toluenesulfonyl chloride; sodium hydroxide;
In
methanol; N,N-dimethyl acetamide; water; dimethyl sulfoxide; acetone;
|
1417576-00-1 Upstream products
-
139481-41-7
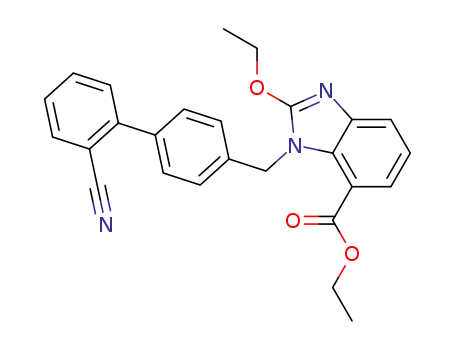
ethyl 1-[(2'-cyano-[1,1']-biphenyl-4-yl)methyl]-2-ethoxy-1H-benzo[d]imidazole-7-carboxylate
-
147403-65-4
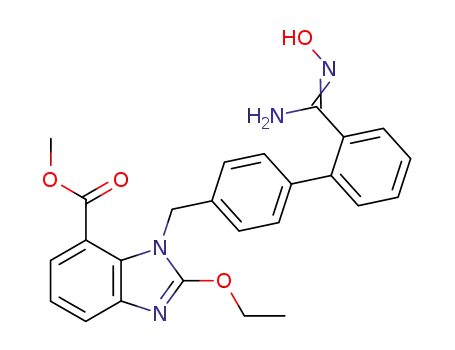
methyl 2-ethoxy-1-((2′-(N′-hydroxycarbamimidoyl)-biphenyl-4-yl)methyl)-1H-benzo[d]imidazole-7-carboxylate
-
1397836-41-7
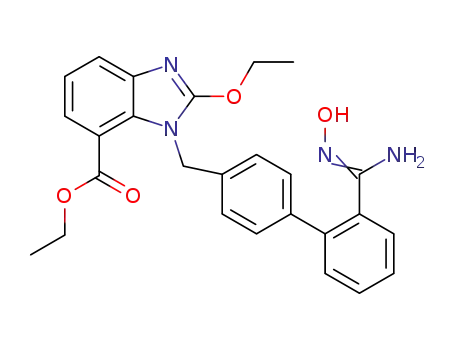
ethyl 2-ethoxy-1-((2′-(N′-hydroxycarbamimidoyl)biphenyl-4-yl)methyl)-1H-benzo[d]imidazole-7-carboxylate
-
147403-52-9
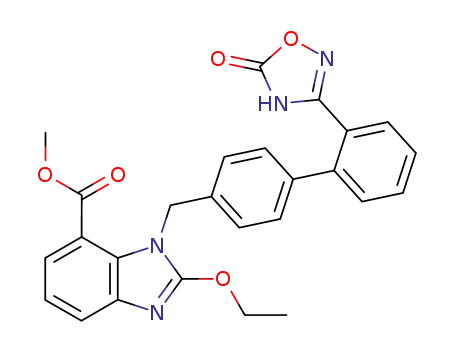
methyl 2-ethoxy-1-((2′-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)biphenyl-4-yl)methyl)-1H-benzo[d]-imidazole-7-carboxylate


