Vitamin B6
-
Product Name :
Vitamin B6
-
CAS No :
65-23-6
-
Project State :
Commercial
Application
General Description
Cost-effective customized wholesale Vitamin B6 65-23-6
- Molecular Formula:C8H11NO3
- Molecular Weight:169.18
- Appearance/Colour:crystalline solid
- Melting Point:214-215 °C(lit.)
- Refractive Index:1.5100 (estimate)
- Boiling Point:491.9 °C at 760 mmHg
- PKA:pKa 5.00(H2O t = 25.0 I = 0.15 (mixed)) (Uncertain)
- Flash Point:251.3 °C
- PSA:73.58000
- Density:1.353 g/cm3
- LogP:0.08020
3,4-Pyridinedimethanol,5-hydroxy-6-methyl-(Cas 65-23-6) Usage
|
Pharmacodynamics |
Vitamin B6 (pyridoxine) is a water-soluble vitamin used in the prophylaxis and treatment of vitamin B6 deficiency and peripheral neuropathy in those receiving isoniazid (isonicotinic acid hydrazide, INH). Vitamin B6 has been found to lower systolic and diastolic blood pressure in a small group of subjects with essential hypertension. Hypertension is another risk factor for atherosclerosis and coronary heart disease. Another study showed pyridoxine hydrochloride to inhibit ADP- or epinephrine-induced platelet aggregation and to lower total cholesterol levels and increase HDL-cholesterol levels, again in a small group of subjects. Vitamin B6, in the form of pyridoxal 5'-phosphate, was found to protect vascular endothelial cells in culture from injury by activated platelets. Endothelial injury and dysfunction are critical initiating events in the pathogenesis of atherosclerosis. |
|
Toxicity |
Oral Rat LD50 = 4 gm/kg. Toxic effects include convulsions, dyspnea, hypermotility, diarrhea, ataxia and muscle weakness. |
|
History |
The discovery of vitamin was tortuous and legendary. After fat-soluble A and water soluble B were discovered by the year of 1915, the discovery of vitamins entered into a rapid developed period. In separation process of riboflavin by Kuhn and his colleagues, they noticed the unusual relationship between growth-promoting activ ity and fluorescence of extracts. Then they supposed that the existence of no fluorescent substances were very necessary for growth-promoting activity of riboflavin. And they considered this phenomenon as the evidence of a second chem ical existence in the thermostable complex. At last, they named this substance as vitamin B6 .Vitamin B6 is widely distributed in foods, including meats, whole-grain products (especially wheat), vegetables, and nuts. In the cereal grains, vitamin B6 is concen trated primarily in the germ and aleuronic layer. Thus, the refining of grains in the production of flours, which removes much of these fractions, results in substantial reductions of vitamin B6 content. The chemical forms of vitamin B6 tend to vary among foods between plant and animal origin: plant tissues contain most pyridox ine (the free alcohol form, pyridoxol), whereas animal tissues contain most pyri doxal and pyridoxamine. |
|
Indications |
Vitamin B6 deficiency |
|
World Health Organization (WHO) |
Pyridoxine (vitamin B6) is listed in theWHO Model List of Essential Drugs. |
|
Biological Activity |
pyridoxine (pyridoxol, vitamin b6, gravidox), also known as vitamin b6, is a form of vitamin b6 found commonly in food and used as dietary supplement. pyridoxine exerts antioxidant effects in cell model of alzheimer's disease via the nrf-2/ho-1 pathway. |
|
Biochem/physiol Actions |
Pyridoxine plays a key role in cell maintenance and amino acid metabolism. Deficiency of vitamin B6 leads to anemia especially in pregnant women and seizures in newborns. It serves as cofactor for heme biosynthesis during δ-amino levulinic acid formation, γ-aminobutyric acid (GABA) transaminase and glutamic acid decarboxylase. Vitamin B6 also helps in reactive oxygen species (ROS) scavenging and helps plants in overcoming the abiotic and biotic stress. |
|
Pharmacology |
The metabolically active form of vitamin B6 is pyridoxal phosphate, which serves as a coenzyme of numerous enzymes, most of which are involved in the metabolism of amino acids. Vitamin B6 functions through the following general mechanisms: decarboxylation, transamination, racemization, elimination, replacement reactions, and β-group interconversions.Pyridoxal phosphate is practically involved in all amino acid metabolism reac tions, such as transaminations, transsulfuration, and selenoamino acid metabolism, in both their biosynthesis and their catabolism. Vitamin B6 also plays an important role in the tryptophan–niacin conversion, histamine synthesis, neurotransmitter syn thesis, and hemoglobin synthesis.Vitamin B6 has two roles in gluconeogenesis, transaminations and glycogen uti lization. It is required for the utilization of glycogen to release glucose by serving as a coenzyme of glycogen phosphorylase. |
|
Safety Profile |
Moderately toxic by ingestion, subcutaneous, intravenous, and intraperitoneal routes. Human systemic effects: ataxia, local anesthetic, paresthesia. When heated to decomposition it emits toxic fumes of Nox |
|
Veterinary Drugs and Treatments |
Pyridoxine use in veterinary medicine is relatively infrequent. It may be of benefit in the treatment of isoniazid (INH) or crimidine (an older rodenticide) toxicity. Pyridoxine deficiency is apparently extremely rare in dogs or cats able to ingest food. Cats with severe intestinal disease may have a greater requirement for pyridoxine in their diet. Experimentally, pyridoxine has been successfully used in dogs to reduce the cutaneous toxicity associated with doxorubicin containing pegylated liposomes (Doxil?). Pyridoxine has been demonstrated to suppress the growth of feline mammary tumors (cell line FRM) in vitro. In humans, labeled uses for pyridoxine include pyridoxine deficiency and intractable neonatal seizures secondary to pyridoxine dependency syndrome. Unlabeled uses include premenstrual syndrome (PMS), carpal tunnel syndrome, tardive dyskinesia secondary to antipsychotic drugs, nausea and vomiting in pregnancy, hyperoxaluria type 1 and oxalate kidney stones, and for the treatment of isoniazid (INH), cycloserine, hydrazine or Gyometra mushroom poisonings. |
|
Physical properties |
It is one kind of B vitamins, containing pyridoxine or pyridoxal or pyridoxamine. Appearance: colorless crystals at room temperature. Solubility: soluble in water and ethanol. Stability: stable in acid liquor but easily destroyed in alkali liquor. Pyridoxol is resistant to high temperature, but pyridoxal and pyridoxamine are not. |
|
Definition |
ChEBI: A hydroxymethylpyridine with hydroxymethyl groups at positions 4 and 5, a hydroxy group at position 3 and a methyl group at position 2. The 4-methanol form of vitamin B6, it is converted intoto pyridoxal phosphate which is a coenzyme f r synthesis of amino acids, neurotransmitters, sphingolipids and aminolevulinic acid. |
|
General Description |
The discovery of vitamin B6 is generally ascribed to Paul Gy?rgy who first realized there was a vitamin that was distinctly different from vitamin B2 in 1934. Pyridoxine (PN) is the C4 hydroxymethyl derivative, pyridoxal (PL) is the C4 formyl derivative and pyridoxamine (PM) is the C4 aminomethyl derivative of 5-(hydroxymethyl)- 2-methylpyridin-3-ol). Each of these are also converted to their corresponding 5'-phosphate derivatives referred to as pyridoxine 5'-phosphate (PNP), pyridoxal 5'-phosphate (PLP), and pyridoxamine 5'-phosphate (PMP), respectively . Because of their ability to interconvert, all are considered active forms of vitamin B6 in vivo. Although PLP is the major coenzyme form, PMP can also function as a coenzyme primarily in aminotransferases. The major metabolite is 4-pyridoxic acid, which is excreted in the urine. |
InChI:InChI=1/C8H11NO3/c1-5-8(12)7(4-11)6(3-10)2-9-5/h2,10-12H,3-4H2,1H3
65-23-6 Relevant articles
Pyridoxamine, a scavenger agent of carbohydrates
Adrover, Miquel,Vilanova, Bartolome,Munoz, Francisco,Donoso, Josefa
, p. 154 - 167 (2007)
Pyridoxamine has been found to inhibit p...
Purification and characterization of pyridoxine 5′-phosphate phosphatase from Sinorhizobium meliloti
Tazoe, Masaaki,Ichikawa, Keiko,Hoshino, Tatsuo
, p. 2277 - 2284 (2005)
Here we report the purification and bioc...
Biosynthesis of vitamin B6 in Rhizobium: in vitro synthesis of pyridoxine from 1-deoxy-D-xylulose and 4-hydroxy-L-threonine.
Tazoe, Masaaki,Ichikawa, Keiko,Hoshino, Tatsuo
, p. 934 - 936 (2002)
Pyridoxine (vitamin B6) in Rhizobium is ...
Environmentally friendly preparation method for vitamin B6
-
, (2019/07/04)
The invention relates to an environmenta...
Preparation method of high content vitamin B6
-
Paragraph 0047-0048, (2019/07/16)
The invention relates to a preparation m...
Method for preparing vitamin B6 by reduction method
-
Paragraph 0020; 0023; 0032; 0033, (2018/09/08)
The invention discloses a method for pre...
Effect of structure of nucleophile and substrate on the quaternization of heterocyclic amines
Zhuravlev,Verolainen,Voronchikhina
experimental part, p. 1025 - 1028 (2011/01/11)
The influence of the nature of the quate...
65-23-6 Process route
-
-
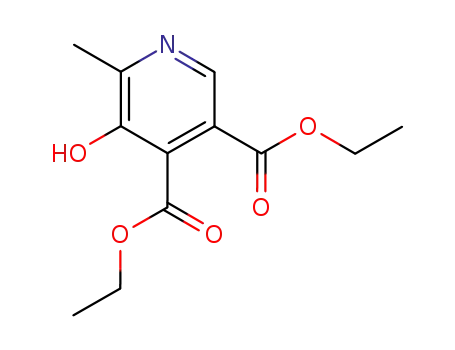
2397-71-9
5-hydroxy-6-methylpyridine-3,4-dicarboxylic acid diethyl ester

-
-
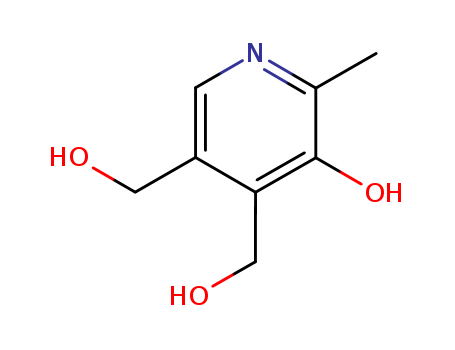
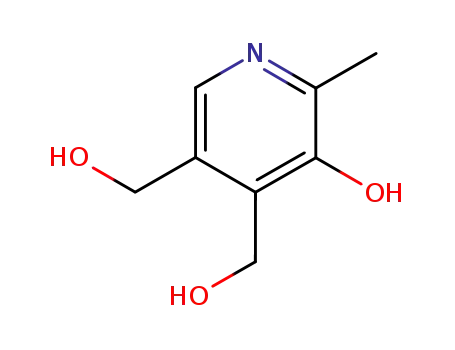
65-23-6
5-hydroxy-6-methyl-3,4-pyridinedimethanol
| Conditions | Yield |
|---|---|
|
With
sodium tetrahydroborate; acetic acid;
In
tetrahydrofuran;
at 30 - 60 ℃;
for 3h;
Solvent;
|
94% |
|
With
Me3SiH(OEt)2; tetrabutyl ammonium fluoride;
In
1,4-dioxane;
at 100 ℃;
for 24h;
|
-
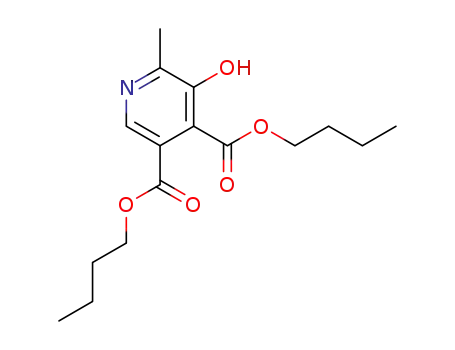
-
butyl 2-methyl-3-hydroxypyridine-4,5-dicarboxylate

-

-
65-23-6
5-hydroxy-6-methyl-3,4-pyridinedimethanol
| Conditions | Yield |
|---|---|
|
With
sodium tetrahydroborate; benzoic acid;
In
tetrahydrofuran;
at 40 - 60 ℃;
for 2h;
Reagent/catalyst;
Solvent;
Temperature;
|
95% |
65-23-6 Upstream products
-
15741-69-2
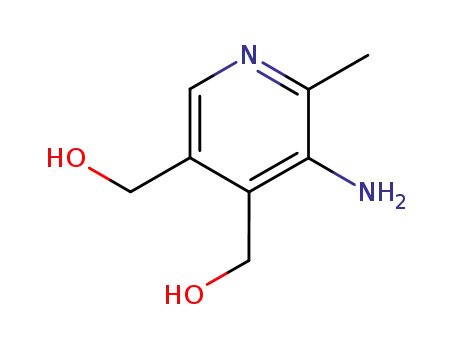
4,5-bis-hydroxymethyl-2-methyl-pyridin-3-ylamine
-
479-30-1
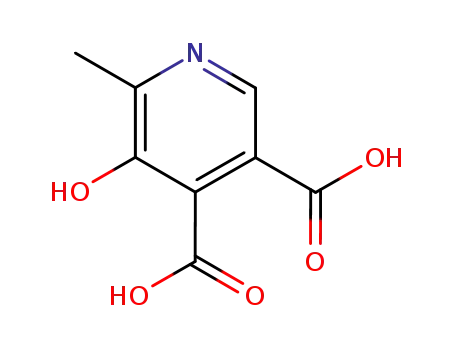
5-hydroxy-6-methyl-pyridine-3,4-dicarboxylic acid
-
18872-74-7

5-hydroxy-6-methyl-pyridine-3,4-dicarboxylic acid dimethyl ester
-
100193-34-8
5-acetoxy-6-methyl-pyridine-3,4-dicarboxylic acid dimethyl ester
65-23-6 Downstream products
-
6273-67-2
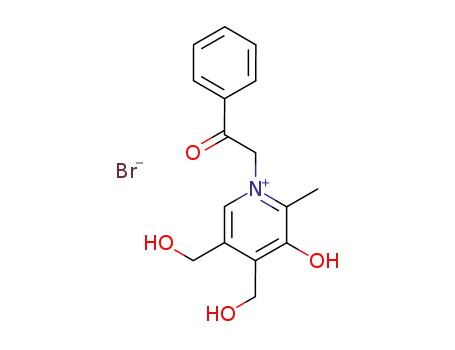
3-hydroxy-4,5-bis-hydroxymethyl-2-methyl-1-phenacyl-pyridinium; bromide
-
61-67-6
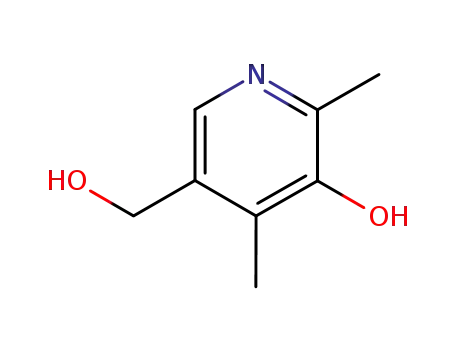
4-deoxypyridoxine
-
6560-46-9
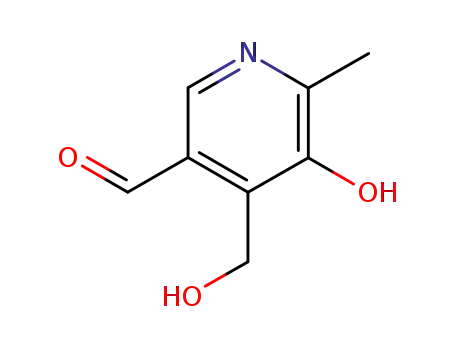
5-hydroxy-4-hydroxymethyl-6-methyl-pyridine-3-carbaldehyde
-
5196-20-3
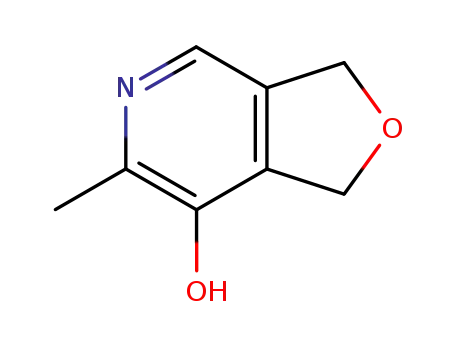
7-hydroxy-6-methyl-1,3-dihydrofuro<3,4-c>pyridin


