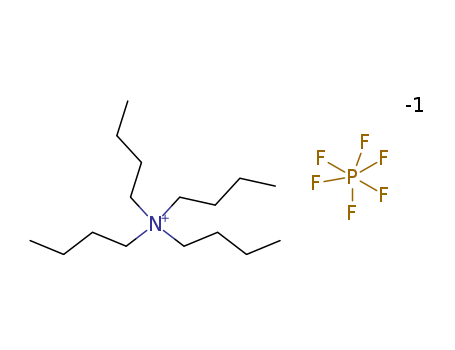
Tetrabutylammonium hexafluorophosphate
-
Product Name :
Tetrabutylammonium hexafluorophosphate
-
CAS No :
3109-63-5
-
Project State :
Commercial
Application
General Description
Buy high quality and low price Tetrabutylammonium hexafluorophosphate 3109-63-5 now
- Molecular Formula:C16H36N.PF6
- Molecular Weight:387.433
- Appearance/Colour:off-white powder
- Melting Point:244-246 ºC
- Boiling Point:242-246 °C
- PSA:13.59000
- LogP:8.38600
Tetrabutylammonium hexafluorophosphate(Cas 3109-63-5) Usage
|
Chemical Description |
Tetrabutylammonium hexafluorophosphate and ferrocene are used as supporting electrolytes and internal standards, respectively, in the electrochemical analysis of the materials. |
|
Purification Methods |
Recrystallise it from saturated EtOH/water and dry it for 10hours in a vacuum at 70o. Also recrystallise it three times from absolute EtOH and dry it for 2 days in a drying pistol under a vacuum at boiling toluene temperature [Bedard & Dahl J Am Chem Soc 108 5933 1986]. It is a stable supporting electrolyte in organic solvents [Baiser in Organic Electrochemitry M. Dekker NY p228 1973.] |
|
General Description |
Visit our Sensor Applications portal to learn more. |
InChI:InChI=1/C16H36N.F6P/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;1-7(2,3,4,5)6/h5-16H2,1-4H3;/q+1;-1
3109-63-5 Relevant articles
Electrochemistry and ion-sensing properties of calix[4]arene derivatives
Chen, Shanshan,Webster, Richard D.,Talotta, Carmen,Troisi, Francesco,Gaeta, Carmine,Neri, Placido
, p. 7036 - 7043 (2010)
The cyclic voltammetric properties of se...
Competing hydrogen-bonding, decomposition, and reversible dimerization mechanisms during the one- and two-electron electrochemical reduction of retinal (Vitamin A)
Tan, Ying Shan,Yue, Yanni,Webster, Richard D.
, p. 9371 - 9379 (2013)
Retinal (R) can be sequentially voltamme...
METAL COMPLEXES WITH TETRAPYRROLE LIGANDS. 50. REDOX POTENTIALS OF SANDWICHLIKE METAL BIS(OCTAETHYLPORPHYRINATES) AND THEIR CORRELATION WITH RING-RING DISTANCES
Buchler, Johann W.,Scharbert, Bernd
, p. 4272 - 4276 (1988)
On the basis of prior work describing th...
Electrochemical/chemical oxidation of bisphenol A in a four-electron/two- proton process in aprotic organic solvents
Chan, Ya Yun,Yue, Yanni,Li, Yongxin,Webster, Richard D.
, p. 287 - 294 (2013)
The electrochemical behavior of bispheno...
Phenylcyanamidoruthenium scorpionate complexes
Harb, Carmen,Kravtsov, Pavel,Choudhuri, Mohommad,Sirianni, Eric R.,Yap, Glenn P.A.,Lever,Crutchley, Robert J.
, p. 1621 - 1630 (2013)
Nine [Ru(Tp)(dppe)L] complexes, where Tp...
PGSE NMR diffusion overhauser studies on [Ru(Cp*)(η6- arene)][PF6], plus a variety of transition-metal, inorganic, and organic salts: An overview of ion pairing in dichloromethane
Moreno, Aitor,Pregosin, Paul S.,Veiros, Luis F.,Albinati, Alberto,Rizzato, Silvia
, p. 5617 - 5629 (2008)
PGSE diffusion, 1F, 1H HOESY and 13CNMR ...
Thermal stability of quaternary ammonium hexafluorophosphates and halides
Zhuravlev,Nikol'skii,Voronchikhina
, p. 824 - 830 (2013)
Thermal decomposition of hexafluorophosp...
Tetracyanido(difluorido)phosphates M+[PF2(CN)4]-
Bresien, Jonas,Ellinger, Stefan,Harloff, J?rg,Schulz, Axel,Sievert, Katharina,Stoffers, Alrik,T?schler, Christoph,Villinger, Alexander,Zur T?schler, Cornelia
, p. 4474 - 4477 (2015)
The systematic study of the reaction of ...
Deoxygenative Fluorination of Phosphine Oxides: A General Route to Fluorinated Organophosphorus(V) Compounds and Beyond
Bornemann, Dustin,Brüning, Fabian,Grützmacher, Hansj?rg,Guan, Liangyu,Küng, Sebastian,Pitts, Cody Ross,Togni, Antonio,Trapp, Nils,Wettstein, Lionel
supporting information, p. 22790 - 22795 (2020/10/06)
Fluorinated organophosphorus(V) compound...
Lewis Acidity Scale of Diaryliodonium Ions toward Oxygen, Nitrogen, and Halogen Lewis Bases
Legault, Claude Y.,Mayer, Robert J.,Mayr, Herbert,Ofial, Armin R.
supporting information, (2020/03/13)
Equilibrium constants for the associatio...
Gold(I) Complexes Nuclearity in Constrained Ferrocenyl Diphosphines: Dramatic Effect in Gold-Catalyzed Enyne Cycloisomerization
Nguyen, Tuan-Anh,Roger, Julien,Nasrallah, Houssein,Rampazzi, Vincent,Fournier, Sophie,Cattey, Hélène,Sosa Carrizo, E. Daiann,Fleurat-Lessard, Paul,Devillers, Charles H.,Pirio, Nadine,Lucas, Dominique,Hierso, Jean-Cyrille
supporting information, p. 2879 - 2885 (2020/08/13)
Di-tert-butylated-bis(phosphino)ferrocen...
Lewis Acid Catalyzed Synthesis of Cyanidophosphates
Bl?sing, Kevin,Ellinger, Stefan,Harloff, J?rg,Schulz, Axel,Sievert, Katharina,T?schler, Christoph,Villinger, Alexander,Zurt?schler, Cornelia
supporting information, p. 4175 - 4188 (2016/03/16)
Salts containing new cyanido(fluorido)ph...
3109-63-5 Process route
-
-
1643-19-2
tetrabutylammomium bromide

-
-
3109-63-5
tert-butylammonium hexafluorophosphate(V)
| Conditions | Yield |
|---|---|
|
With
trimethyl phosphite; hexafluorophosphoric acid;
at 0 - 60 ℃;
for 15h;
Inert atmosphere;
neat (no solvent);
|
96% |
|
With
potassium hexafluorophosphate;
In
dichloromethane; water;
at 20 ℃;
for 24h;
|
88% |
|
With
potassium hexafluorophosphate;
In
dichloromethane; water;
at 20 ℃;
for 24h;
|
88% |
|
With
potassium hexafluorophosphate;
In
dichloromethane; water;
at 25 ℃;
for 24h;
|
88% |
|
With
ammonium hexafluorophosphate;
In
acetone;
Heating;
|
|
|
With
potassium hexafluorophosphate;
|
|
|
With
hexafluorophosphoric acid;
|
|
|
With
potassium hexafluorophosphate;
In
water;
|
-
-
1112-67-0
tetrabutyl-ammonium chloride

-
-
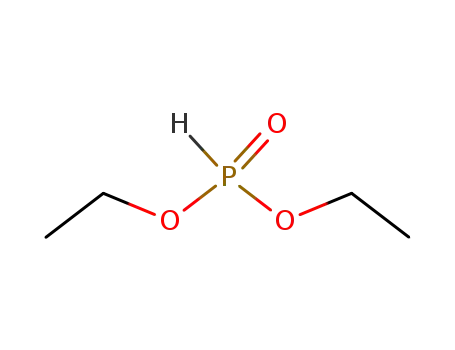
762-04-9
phosphonic acid diethyl ester

-
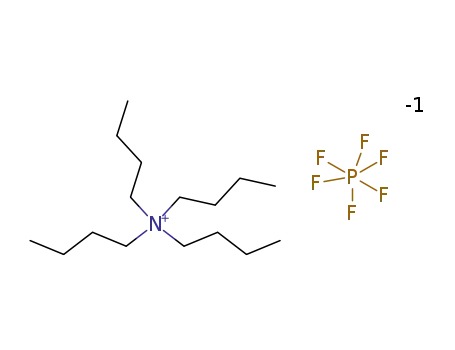
-
3109-63-5
tert-butylammonium hexafluorophosphate(V)
| Conditions | Yield |
|---|---|
|
phosphonic acid diethyl ester;
With
potassium fluoride; oxalyl dichloride;
In
acetonitrile;
at 20 ℃;
Glovebox;
Sealed tube;
Inert atmosphere;
tetrabutyl-ammonium chloride;
In
water;
at 20 ℃;
for 0.166667h;
Glovebox;
Sealed tube;
Inert atmosphere;
|
59% |
3109-63-5 Upstream products
-
1643-19-2
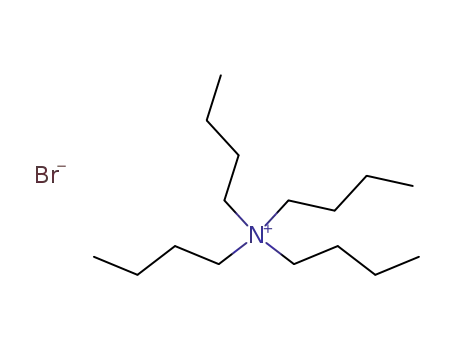
tetrabutylammomium bromide
-
67-56-1
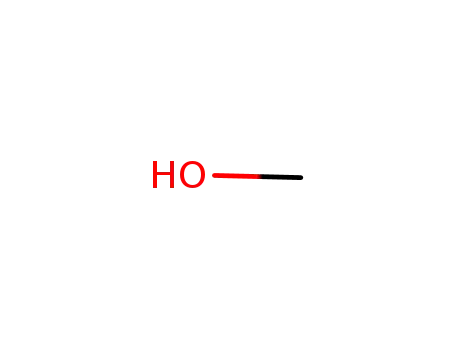
methanol
-
2052-49-5
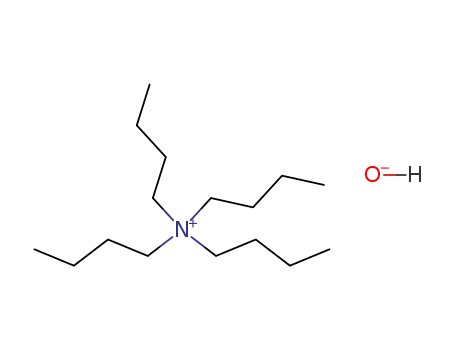
tetra(n-butyl)ammonium hydroxide
-
17084-13-8
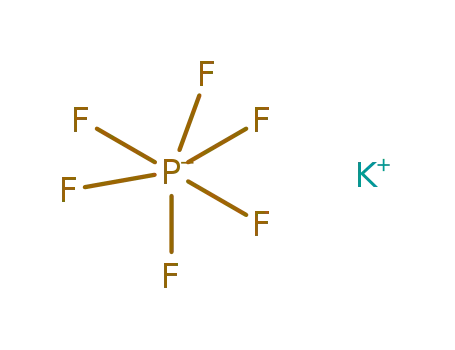
potassium hexafluorophosphate
3109-63-5 Downstream products
-
125357-14-4

C16H36N(1+)*C14H12(1-)
-
125341-42-6

C16H36N(1+)*C16H14(1-)
-
125341-43-7

C18H16(1-)*C16H36N(1+)
-
114490-26-5
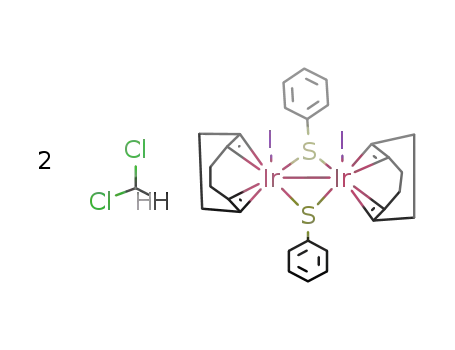
Ir2I2(μ2-SPh)2(COD)2*2CH2Cl2


