Dibenzofuran, 1-nitro-
-
Product Name :
Dibenzofuran, 1-nitro-
-
CAS No :
87812-99-5
-
Project State :
Commercial
Application
General Description
Cost-effective and customizable Dibenzofuran, 1-nitro- 87812-99-5 factory
- Molecular Formula:C12H7NO3
- Molecular Weight:213.192
- Vapor Pressure:1.26E-05mmHg at 25°C
- Boiling Point:379.6°C at 760 mmHg
- Flash Point:183.4°C
- PSA:58.96000
- Density:1.399g/cm3
- LogP:4.01740
87812-99-5 Relevant articles
Tetramethylammonium fluoride tetrahydrate-mediated transition metal-free coupling of aryl iodides with unactivated arenes in air
Nozawa-Kumada, Kanako,Nakamura, Kosuke,Kurosu, Satoshi,Iwakawa, Yuki,Denneval, Charline,Shigeno, Masanori,Kondo, Yoshinori
, p. 1042 - 1045 (2019/10/02)
Biaryls are important compounds with wid...
An aromatic heterocyclic compound and use thereof
-
Paragraph 0231; 0234-0237, (2019/11/04)
The invention provides an aromatic heter...
Preparation method of 1-iododibenzofuran
-
Paragraph 0022-0024; 0027-0029, (2019/08/03)
The invention provides a preparation met...
PHOSPHORESCENT EMITTERS
-
, (2011/09/16)
Compounds including a ligand with a dibe...
87812-99-5 Process route
-
-
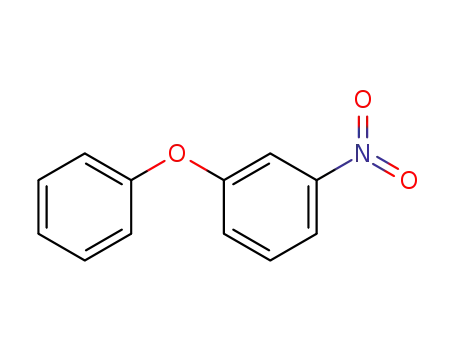
620-55-3
1-nitro-3-phenoxybenzene

-
-
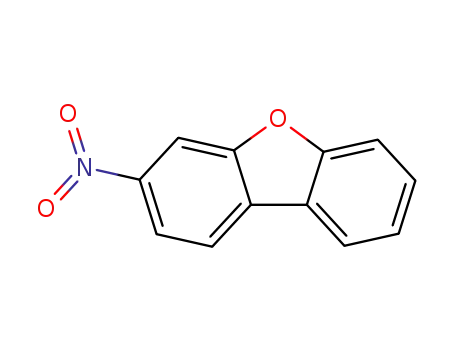
5410-97-9
3-nitrodibenzofuran

-
-
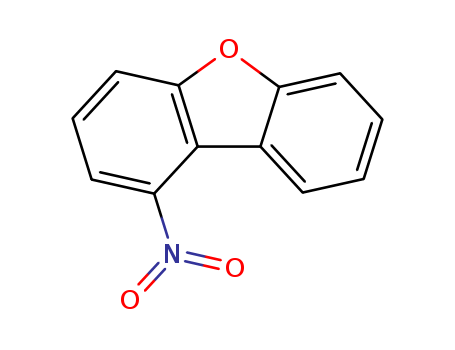
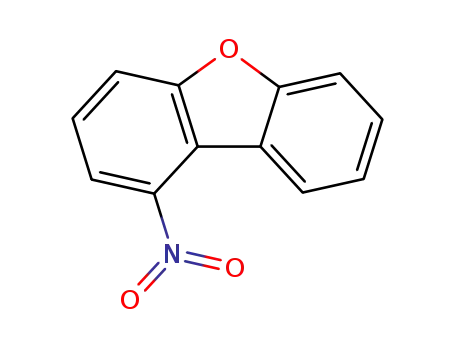
87812-99-5
1-nitrodibenzofuran
| Conditions | Yield |
|---|---|
|
With
palladium diacetate;
In
trifluoroacetic acid;
at 70 ℃;
for 1h;
|
44% |
-
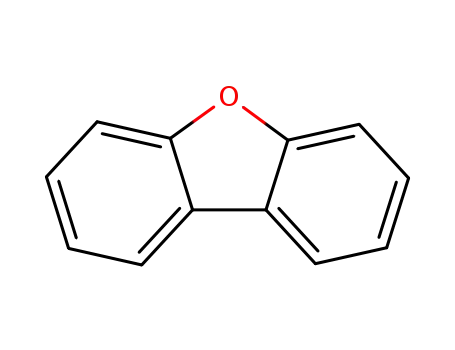
-
132-64-9,214827-48-2
dibenzofuran

-

-
5410-97-9
3-nitrodibenzofuran

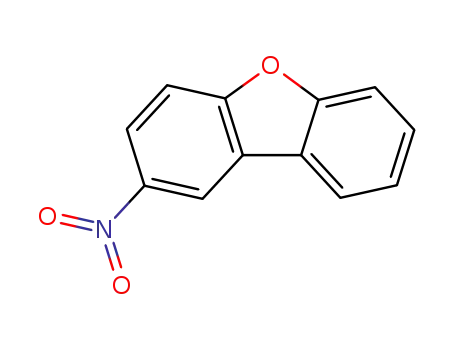
-
-
20927-95-1
2-nitrodibenzofuran

-
![4-nitrodibenzo[b,d]furan](/upload/2025/9/3cedce5f-e51d-443d-97c8-93ab36210d56.png)
-
86607-81-0
4-nitrodibenzo[b,d]furan

-

-
87812-99-5
1-nitrodibenzofuran
| Conditions | Yield |
|---|---|
|
With
tetranitromethane;
In
trifluoroacetic acid;
at 0 ℃;
for 10h;
Product distribution;
Mechanism;
Irradiation;
-45 deg. C, also in dark; regioselectivity studied;
|
6% 11% 83% 1% |
|
With
nitric acid; trifluoroacetic acid;
at 0 ℃;
for 0.5h;
|
80% |
|
With
aluminium trichloride; ethyl nitrate;
In
nitromethane;
at 25 ℃;
for 1h;
Further byproducts given;
|
17% 5% 28% |
|
With
tetranitromethane;
In
trifluoroacetic acid;
at 0 ℃;
for 10h;
Product distribution;
Mechanism;
Irradiation;
positional reactivity of dibenzofuran; charge-transfer nitration; other solvents;
|
6 % Chromat. 11 % Chromat. 83 % Chromat. 1 % Chromat. |
|
With
sodium azide; sulfuric acid; sodium nitrite;
In
acetic acid;
at 20 ℃;
for 20h;
Product distribution;
var. reagents, solvents, temp.;
|
87812-99-5 Upstream products
-
132-64-9

dibenzofuran
-
99-65-0
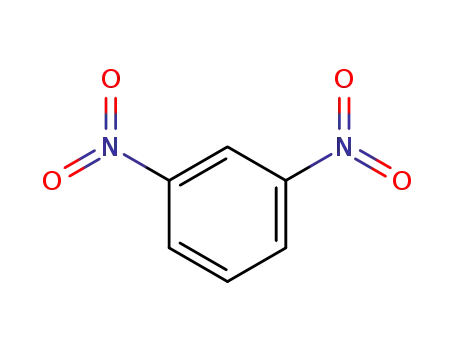
meta-dinitrobenzene
-
533-58-4
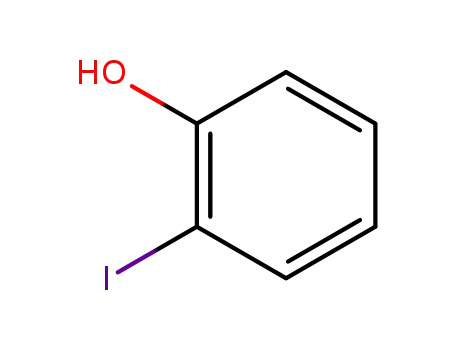
2-Iodophenol
-
104-82-5

4-Methylbenzyl chloride
87812-99-5 Downstream products
-
1332882-07-1
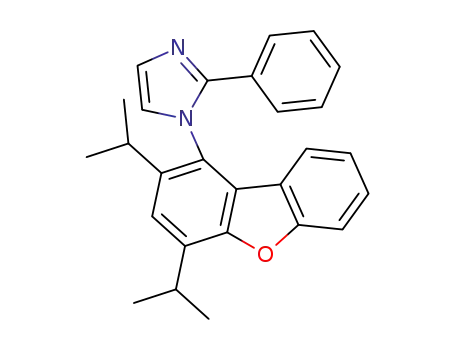
1-(2,4-diisopropyldibenzo[b,d]furan-1-yl)-2-phenyl-1H-imidazole
-
50548-40-8
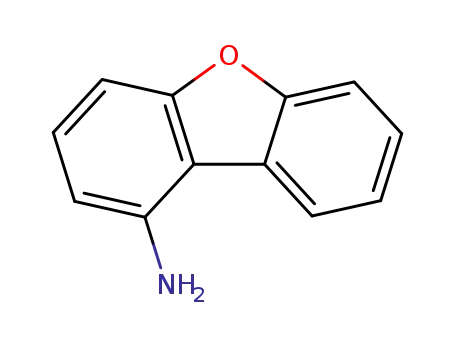
1-aminodibenzofuran


