9-Fluorenemethanol
-
Product Name :
9-Fluorenemethanol
-
CAS No :
24324-17-2
-
Project State :
Commercial

Application
General Description
Cost-effective and customizable 9-Fluorenemethanol 24324-17-2 in stock
- Molecular Formula:C14H12O
- Molecular Weight:196.249
- Appearance/Colour:white to pale yellow crystalline powder
- Vapor Pressure:6.2E-06mmHg at 25°C
- Melting Point:105-107 °C(lit.)
- Refractive Index:1.638
- Boiling Point:363.9 °C at 760 mmHg
- PKA:13.68±0.10(Predicted)
- Flash Point:167.4 °C
- PSA:20.23000
- Density:1.175 g/cm3
- LogP:2.79120
9-Fluorenemethanol(Cas 24324-17-2) Usage
|
General Description |
Vibrational spectroscopy of jet-cooled 9-fluorenemethanol and its clusters 9-fluorenemethanol-H2O, 9-fluorenemethanol-CH3OH, 9-fluorenemethanol-C2H5OH and 9-fluorenemethanol-C3H7OH has been studied by IR-UV double-resonance method. Electropolymerization of 9-fluorenemethanol in boron trifluoride diethyl etherate leads to low-potential electrodeposition of semiconducting poly(9-fluorenemethanol) (PFMO) film. |
InChI:InChI=1/C14H12O/c15-9-14-12-7-3-1-5-10(12)11-6-2-4-8-13(11)14/h1-8,14-15H,9H2
24324-17-2 Relevant articles
Formation and verification of the structure of the 1-fluorenylmethyl chloroformative derivative of sulfamethazine
Liang,Zhang,Baker,Cross
, p. 86 - 92 (1996)
Sulfamethazine (SMZ) is derivatized with...
Application of the Curtius rearrangement to the synthesis of 1′-aminoferrocene-1-carboxylic acid derivatives
Erb, William,Levanen, Gael,Roisnel, Thierry,Dorcet, Vincent
, p. 3808 - 3818 (2018)
The shortest synthesis of N-protected 1′...
-
Brown,Bluestein
, (1943)
-
SAR of novel biarylmethylamine dopamine D4 receptor ligands
Arlt, Michael,Boettcher, Henning,Riethmueller, Angelika,Schneider, Guenter,Bartoszyk, Gerd D.,Greiner, Hartmut,Seyfried, Christoph A.
, p. 2033 - 2038 (1998)
SAR for a novel series of dopamine D4 re...
Me3SI-promoted chemoselective deacetylation: a general and mild protocol
Gurawa, Aakanksha,Kashyap, Sudhir,Kumar, Manoj
, p. 19310 - 19315 (2021/06/03)
A Me3SI-mediated simple and efficient pr...
Method for high-selectivity synthesis of 9-fluorenylcarbinol
-
Paragraph 0025-0080, (2021/06/06)
The invention discloses a method for hig...
Preparation method of 9-hydroxymethyl-fluorene diacid
-
Paragraph 0039-0045; 0062-0063; 0068-0069; 0074-0075, (2020/12/10)
The invention provides a method for prep...
KMnO4-catalyzed chemoselective deprotection of acetate and controllable deacetylation-oxidation in one pot
Gurawa, Aakanksha,Kumar, Manoj,Rao, Dodla S.,Kashyap, Sudhir
supporting information, p. 16702 - 16707 (2020/10/27)
A novel and efficient protocol for chemo...
24324-17-2 Process route
-
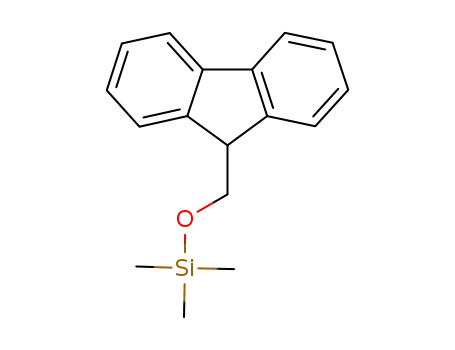
-
1162648-59-0
(9H-fluoren-9-ylmethoxy)trimethylsilane

-
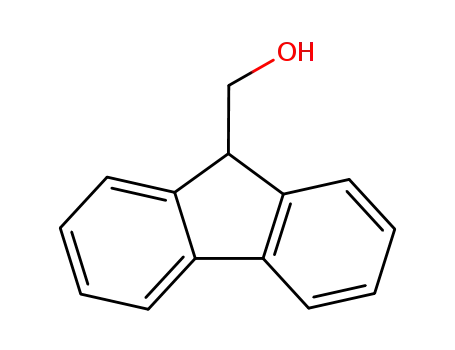
-
24324-17-2
9-Fluorenylmethanol
| Conditions | Yield |
|---|---|
|
With
methanol; 1,3-disulfonic acid imidazolium hydrogen sulfate;
at 20 ℃;
for 0.0833333h;
Green chemistry;
|
99% |
|
With
rice husk ash supported on anatase-phase titania nanoparticles nanocomposite;
In
methanol;
at 20 ℃;
for 0.05h;
|
94% |
|
With
methanol; vanadium hydrogen sulfate;
at 20 ℃;
for 0.0833333h;
|
92% |
|
With
poly (ethylene glycol)-sulfonated sodium montmorillonite nanocomposite;
In
methanol;
at 20 ℃;
for 0.133333h;
|
91% |
|
With
methanol;
at 20 ℃;
for 0.0833333h;
|
85% |
-
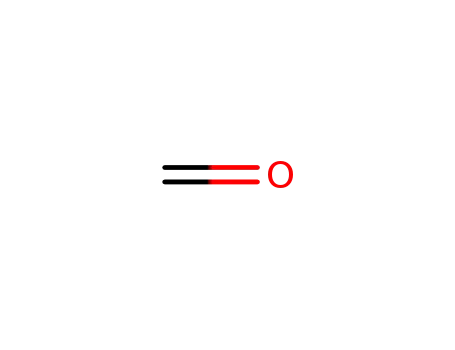
-
50-00-0,30525-89-4,61233-19-0
formaldehyd

-
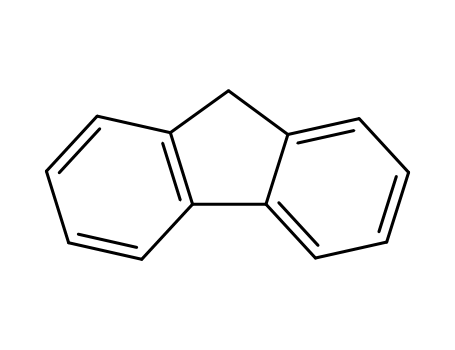
-
86-73-7
9H-fluorene

-

-
24324-17-2
9-Fluorenylmethanol
| Conditions | Yield |
|---|---|
|
9H-fluorene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at 0 ℃;
formaldehyd;
In
tetrahydrofuran; hexane;
at 0 - 20 ℃;
for 5h;
|
71% |
|
9H-fluorene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at 0 ℃;
for 0.133333h;
formaldehyd;
In
tetrahydrofuran; hexane;
at 0 - 20 ℃;
for 1.5h;
|
30.5% |
|
With
1,8-diazabicyclo[5.4.0]undec-7-ene;
In
dimethyl sulfoxide;
at 20 ℃;
for 0.333333h;
Solvent;
|
17% |
|
With
N-benzyl-trimethylammonium hydroxide;
In
pyridine;
|
24324-17-2 Upstream products
-
67-56-1
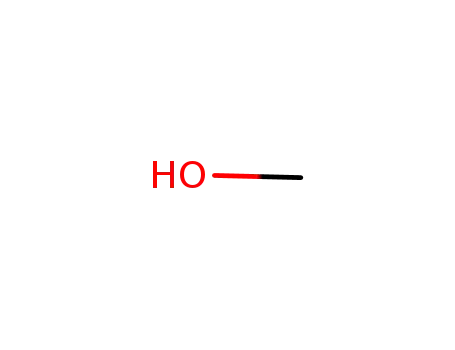
methanol
-
832-80-4
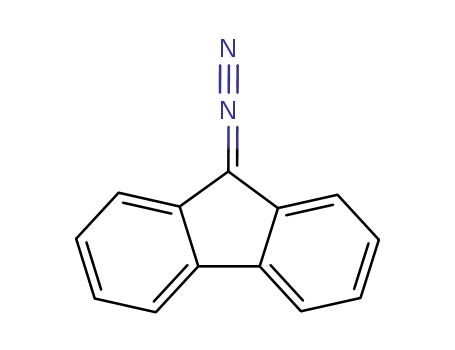
9-diazofluorenone
-
50-00-0
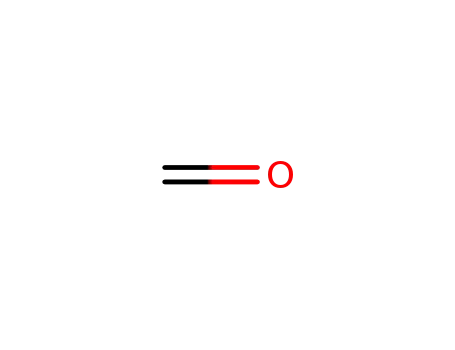
formaldehyd
-
86-73-7

9H-fluorene
24324-17-2 Downstream products
-
85-01-8
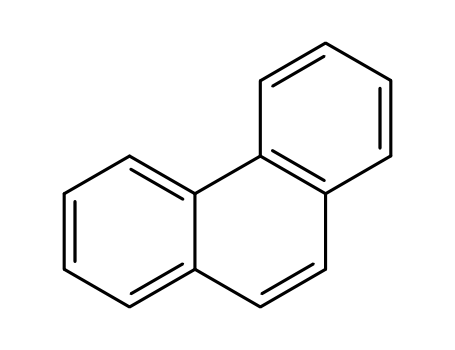
phenanthrene
-
85055-70-5
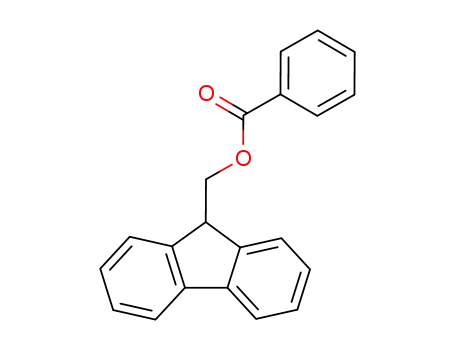
(9H-fluoren-9-yl)methyl benzoate
-
71532-40-6
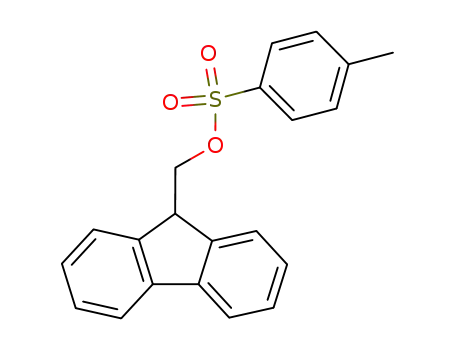
(9H-fluoren-9-yl)methyl 4-methylbenzenesulfonaye
-
28920-43-6
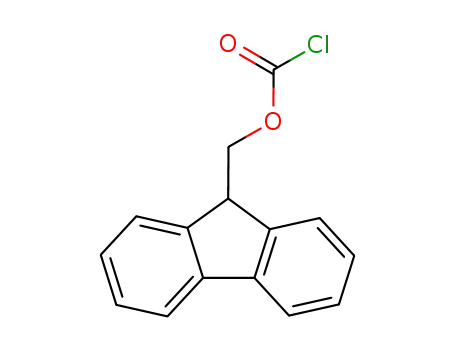
(fluorenylmethoxy)carbonyl chloride


