2-(4-ethyl-3-iodophenyl)-2-Methylpropanoic acid
-
Product Name :
2-(4-ethyl-3-iodophenyl)-2-Methylpropanoic acid
-
CAS No :
1256584-73-2
-
Project State :
Commercial
Application
General Description
Buy cost-effective 99% pure 2-(4-ethyl-3-iodophenyl)-2-Methylpropanoic acid 1256584-73-2 now
- Molecular Formula:C12H15IO2
- Molecular Weight:318.154
- Boiling Point:369.6±27.0 °C(Predicted)
- PKA:4.32±0.11(Predicted)
- PSA:37.30000
- Density:1.547±0.06 g/cm3(Predicted)
- LogP:3.21580
1256584-73-2 Relevant articles
Development of Alectinib-Based PROTACs as Novel Potent Degraders of Anaplastic Lymphoma Kinase (ALK)
Xie, Shaowen,Sun, Yuan,Liu, Yulin,Li, Xinnan,Li, Xinuo,Zhong, Wenyi,Zhan, Feiyan,Zhu, Jingjie,Yao, Hong,Yang, Dong-Hua,Chen, Zhe-Sheng,Xu, Jinyi,Xu, Shengtao
, p. 9120 - 9140 (2021)
A series of novel anaplastic lymphoma ki...
Preparation method and intermediate of halogenated aromatic hydrocarbon compound
-
Paragraph 0145-0147, (2020/07/14)
The invention discloses a preparation me...
Synthesis method of alectinib hydrochloride intermediate 2-(4-ethyl-3-iodophenyl)-2-methylpropanoic acid
-
Paragraph 0062-0064; 0070-0072; 0078-0080; 0086-0088; 0096, (2020/12/29)
The invention discloses a synthesis meth...
Preparation method of aromatic hydrocarbon compound
-
, (2020/12/30)
The invention relates to a preparation m...
1256584-73-2 Process route
-
-
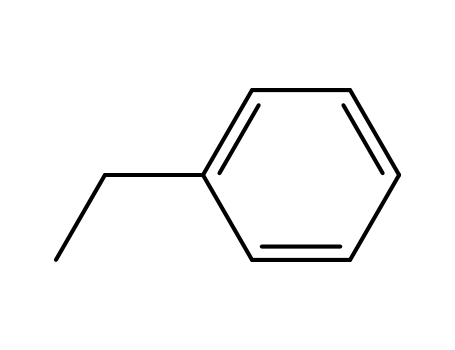
100-41-4,27536-89-6
ethylbenzene

-
-
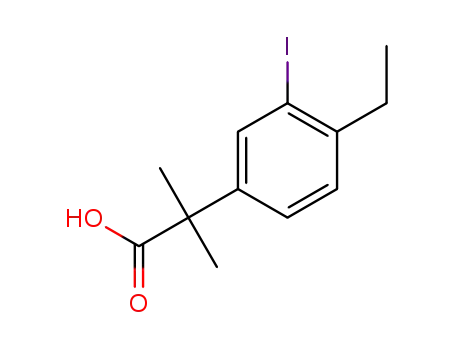
1256584-73-2
2-(4-ethyl-3-iodophenyl)-2-methylpropanoic acid
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 4 steps
1: aluminum (III) chloride / 4-(dicyanomethylene)-2-methyl-6-(p-dimethylaminostyryl)-4H-pyran / -10 °C
2: zinc dibromide / 95 °C
3: sodium hydroxide; water / methanol / Reflux
4: N-iodo-succinimide; methanesulfonic acid / acetonitrile / 15 - 25 °C
With
aluminum (III) chloride; N-iodo-succinimide; methanesulfonic acid; water; sodium hydroxide; zinc dibromide;
In
methanol; 4-(dicyanomethylene)-2-methyl-6-(p-dimethylaminostyryl)-4H-pyran; acetonitrile;
|
|
|
Multi-step reaction with 4 steps
1.1: aluminum (III) chloride; iodine / 15 h / 20 - 66 °C
2.1: ethylmagnesium bromide; magnesium / 7 h / 20 °C / Reflux
2.2: 4 h / 0 - 30 °C
3.1: acetic acid; sodium acetate; sodium hypochlorite solution; sodium chlorite; TEMPO / acetonitrile; water / 12 h / 0 - 30 °C / pH 6.5
4.1: acetic acid; sodium nitrite; sulfuric acid; iodine / 6 h / 0 - 20 °C
With
aluminum (III) chloride; sodium hypochlorite solution; sodium chlorite; TEMPO; sulfuric acid; ethylmagnesium bromide; iodine; sodium acetate; magnesium; acetic acid; sodium nitrite;
In
water; acetonitrile;
|
|
|
Multi-step reaction with 4 steps
1.1: hydrogen fluoride; iodine / 13 h / 20 - 52 °C
2.1: methylmagnesium bromide; magnesium / diethyl ether / 5.3 h / 20 °C / Reflux
2.2: 4 h / 0 - 30 °C
3.1: acetic acid; sodium acetate; sodium hypochlorite solution; sodium chlorite; TEMPO / acetonitrile; water / 12 h / 0 - 30 °C / pH 6.5
4.1: acetic acid; sodium nitrite; sulfuric acid; iodine / 6 h / 0 - 20 °C
With
sodium hypochlorite solution; sodium chlorite; TEMPO; sulfuric acid; hydrogen fluoride; methylmagnesium bromide; iodine; sodium acetate; magnesium; acetic acid; sodium nitrite;
In
diethyl ether; water; acetonitrile;
|
|
|
Multi-step reaction with 4 steps
1.1: sulfuric acid / 5 h / 10 - 40 °C
2.1: iodine; magnesium / 2-methyltetrahydrofuran; ethylene dibromide / 3.6 h / 20 °C / Reflux
2.2: 4 h / 0 - 30 °C
3.1: acetic acid; sodium acetate; sodium hypochlorite solution; sodium chlorite; TEMPO / acetonitrile; water / 12 h / 0 - 30 °C / pH 6.5
4.1: acetic acid; sodium nitrite; sulfuric acid; iodine / 6 h / 0 - 20 °C
With
sodium hypochlorite solution; sodium chlorite; TEMPO; sulfuric acid; iodine; sodium acetate; magnesium; acetic acid; sodium nitrite;
In
2-methyltetrahydrofuran; water; ethylene dibromide; acetonitrile;
|
-
-
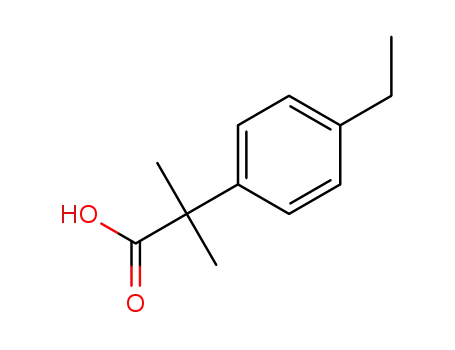
1247119-83-0
2-(4-ethylphenyl)-2-methyl-propanoic acid

-

-
1256584-73-2
2-(4-ethyl-3-iodophenyl)-2-methylpropanoic acid
| Conditions | Yield |
|---|---|
|
With
N-iodo-succinimide; sulfuric acid; acetic acid;
at 0 - 30 ℃;
|
90% |
|
With
N-iodo-succinimide; sulfuric acid; acetic acid;
at 0 - 20 ℃;
for 2h;
|
90% |
|
With
N-iodo-succinimide; methanesulfonic acid;
In
acetonitrile;
at 15 - 25 ℃;
|
85% |
|
With
sulfuric acid; iodine; acetic acid; sodium nitrite;
at 0 - 20 ℃;
for 6h;
Temperature;
Reagent/catalyst;
|
84.2% |
|
With
sulfuric acid; iodine; acetic acid; periodic acid;
In
tetrahydrofuran; water;
at 75 ℃;
for 6h;
|
84% |
1256584-73-2 Upstream products
-
1247119-83-0

2-(4-ethylphenyl)-2-methyl-propanoic acid
-
100-41-4

ethylbenzene
-
698394-60-4
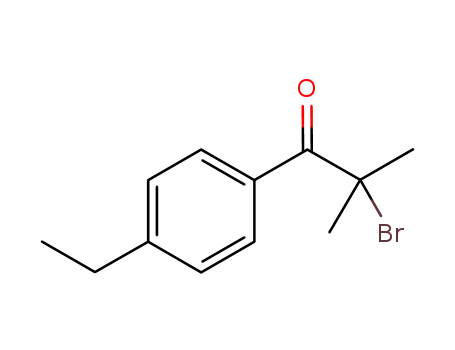
2-bromo-1-(4-ethylphenyl)-2-methyl-propan-1-one
-
698394-59-1
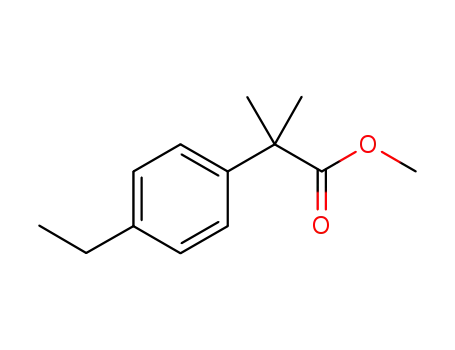
methyl 2-(4-ethylphenyl)-2-methyl-propanoate
1256584-73-2 Downstream products
-
1256580-46-7
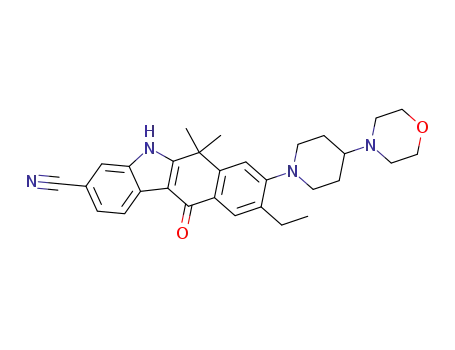
C(248)H5402


