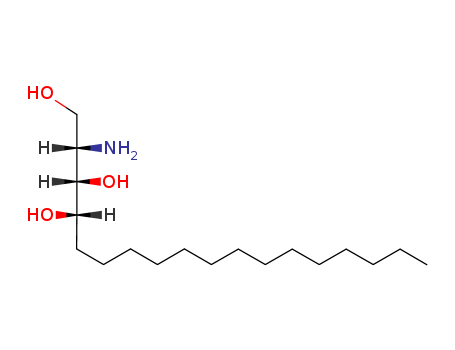
phytosphingosine
-
Product Name :
phytosphingosine
-
CAS No :
13552-11-9
-
Project State :
Commercial
Application
General Description
Cost-effective and customizable phytosphingosine 13552-11-9 factory
- Molecular Formula:C18H39NO3
- Molecular Weight:317.513
- Melting Point:136-138 °C(Solv: acetonitrile (75-05-8))
- Boiling Point:483.7±40.0 °C(Predicted)
- PKA:11.91±0.45(Predicted)
- PSA:86.71000
- Density:0.983±0.06 g/cm3(Predicted)
- LogP:3.81930
13552-11-9 Relevant articles
Development of an Amino Acid/Hydroxy Oxime Dual Catalyst System for Highly Stereoselective Direct Asymmetric Aldol Reactions in the Presence of Water
Mridha, Moniruzzaman,Ma, Guangning,Palo-Nieto, Carlos,Afewerki, Samson,Cordova, Armando
, p. 383 - 390 (2016/12/24)
An eco-friendly dual catalyst system for...
Chemo- and Diastereoselective N-Heterocyclic Carbene-Catalyzed Cross-Benzoin Reactions Using N-Boc-α-amino Aldehydes
Haghshenas, Pouyan,Gravel, Michel
supporting information, p. 4518 - 4521 (2016/09/28)
N-Boc-α-amino aldehydes are shown to be ...
Total synthesis of d - Lyxo -phytosphingosine and formal synthesis of pachastrissamine via a chiral 1,3-oxazine
Mu, Yu,Kim, Ji-Yeon,Jin, Xiangdan,Park, Seok-Hwi,Joo, Jae-Eun,Ham, Won-Hun
experimental part, p. 2340 - 2346 (2012/09/07)
Concise and efficient syntheses of d-lyx...
Total syntheses of D -xylo- and D -arabino-phytosphingosine based on the syntheses of chiral 1,3-oxazines
Mu, Yu,Jin, Tian,Kim, Gun-Woo,Kim, Jin-Seok,Kim, Sung-Soo,Tian, Yong-Shou,Oh, Chang-Young,Ham, Won-Hun
experimental part, p. 2614 - 2620 (2012/07/13)
An efficient, stereocontrolled, and shor...
13552-11-9 Process route
-
-
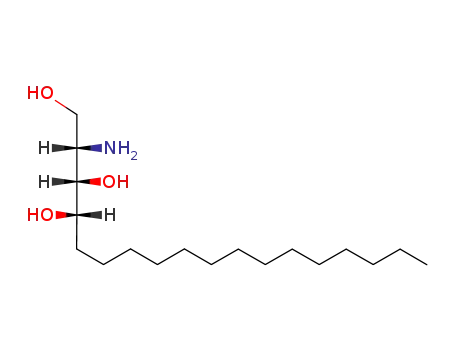
C35H61NO7

-
-
554-62-1,1987-39-9,13552-11-9,21436-10-2,21436-11-3,22565-80-6,22565-81-7,67337-52-4,111466-48-9
(2S,3S,4S)-2-aminooctadecane-1,3,4-triol
| Conditions | Yield |
|---|---|
|
With
lithium hydroxide;
In
tetrahydrofuran;
at 22 ℃;
for 2h;
|
98% |
-
-
C36H81NO3Si3

-

-
554-62-1,1987-39-9,13552-11-9,21436-10-2,21436-11-3,22565-80-6,22565-81-7,67337-52-4,111466-48-9
(2S,3S,4S)-2-aminooctadecane-1,3,4-triol
| Conditions | Yield |
|---|---|
|
With
tetrabutyl ammonium fluoride;
In
tetrahydrofuran;
at 20 ℃;
for 24h;
|
68% |
13552-11-9 Upstream products
-
111394-76-4
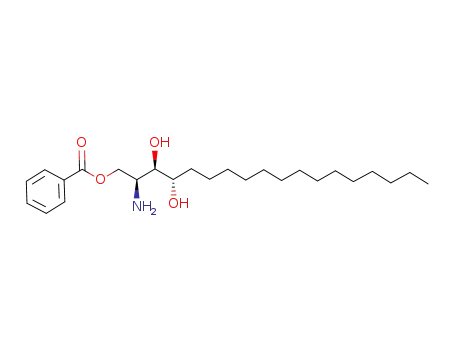
(2S,3R,4S)-2-amino-1-benzoyloxy-octadecane-3,4-diol
-
214556-72-6
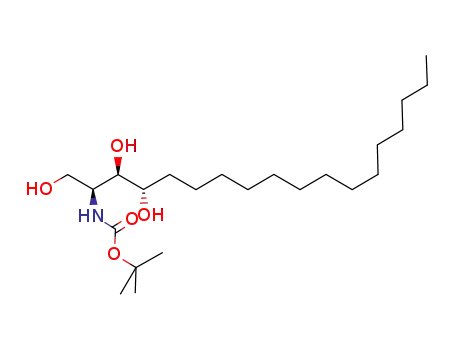
tert-butyl [(2S,3R,4S)-1,3,4-trihydroxyoctadecan-2-yl]carbamate
-
253787-68-7
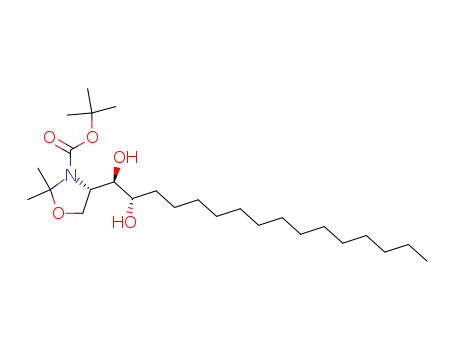
(S)-tert-butyl 4-[(1R,2S)-1,2-dihydroxyhexadecyl]-2,2-dimethyloxazolidine-3-carboxylate
-
866832-30-6
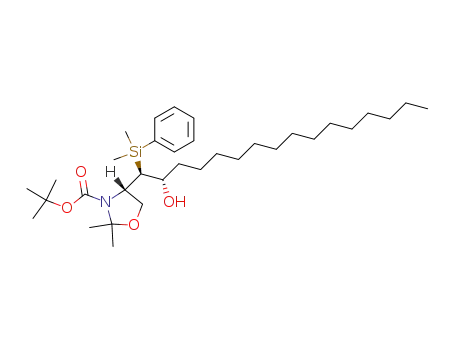
(S)-4-[(1R,2S)-1-(Dimethyl-phenyl-silanyl)-2-hydroxy-hexadecyl]-2,2-dimethyl-oxazolidine-3-carboxylic acid tert-butyl ester


