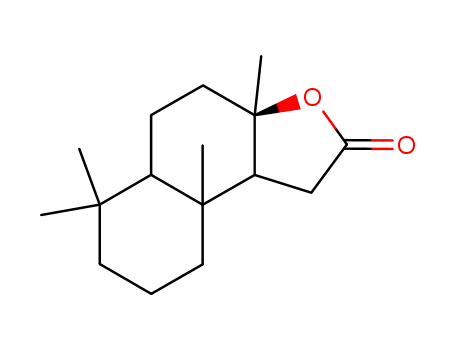
Sclareolide
-
Product Name :
Sclareolide
-
CAS No :
564-20-5
-
Project State :
Commercial
Application
General Description
High quality purity >99% Sclareolide 564-20-5 for sale
- Molecular Formula:C16H26O2
- Molecular Weight:250.381
- Appearance/Colour:off-white to white crystal powder
- Vapor Pressure:0.000299mmHg at 25°C
- Melting Point:124-126 °C
- Refractive Index:1.489
- Boiling Point:321.4 °C at 760 mmHg
- Flash Point:132.4 °C
- PSA:26.30000
- Density:1.009 g/cm3
- LogP:3.93460
Sclareolide(Cas 564-20-5) Usage
|
Preparation |
Sclareol, isolated from clary sage by solvent extraction, can be efficiently oxidized by the microorganism Cryptococcus albidus to sclareolide. |
|
Flammability and Explosibility |
Notclassified |
|
General Description |
Sclareolide is a diterpenoid compound mainly used in the perfume industry for its amber like odor. |
InChI:InChI=1/C16H26O2/c1-14(2)7-5-8-15(3)11(14)6-9-16(4)12(15)10-13(17)18-16/h11-12H,5-10H2,1-4H3/t11-,12+,15-,16+/m0/s1
564-20-5 Relevant articles
Catalytic Highly Regioselective C-H Oxygenation Using Water as the Oxygen Source: Preparation of 17O/18O-Isotope-Labeled Compounds
Doiuchi, Daiki,Uchida, Tatsuya
supporting information, p. 7301 - 7305 (2021/10/01)
We found that the oxygen atom of water i...
Palladium-Catalyzed Low Pressure Carbonylation of Allylic Alcohols by Catalytic Anhydride Activation
Schelwies, Mathias,Paciello, Rocco,Pelzer, Ralf,Siegel, Wolfgang,Breuer, Michael
, p. 9263 - 9266 (2021/05/27)
A direct carbonylation of allylic alcoho...
Chiral complementary alkyl heterocyclic compounds and their use as fungicides
-
Paragraph 0060-0064, (2020/10/20)
The invention relates to a chiral driman...
METHOD FOR PRODUCING SCLAREOLIDE
-
Paragraph 0027; 0028, (2020/12/30)
A method for producing slcareolide compr...
564-20-5 Process route
-
-
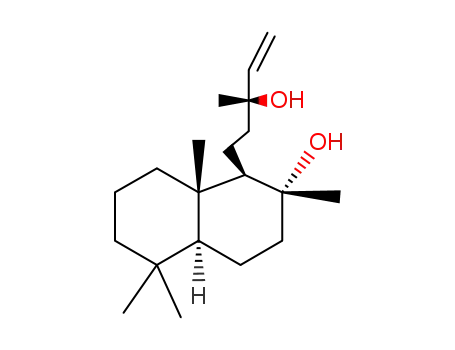
515-03-7
sclareol

-
-
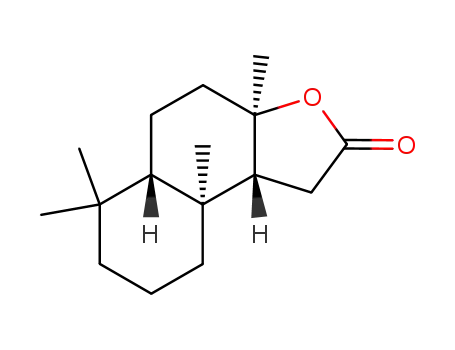
564-20-5,1216-84-8,30450-17-0,58976-28-6,64090-92-2,67844-42-2,79768-41-5,79768-42-6,86688-27-9,86688-28-0,95406-08-9,98719-44-9,98719-46-1,131831-65-7,140851-80-5
(3aR)-(+)-sclareolide
| Conditions | Yield |
|---|---|
|
With
cyclooxygenase; sulfuric acid; dihydrogen peroxide;
In
1,2-dichloro-ethane;
at 50 ℃;
for 4h;
pH=2;
Time;
Enzymatic reaction;
|
83.4% |
|
With
potassium permanganate; acetic anhydride;
In
acetone;
at 0 - 20 ℃;
|
83% |
|
sclareol;
With
potassium permanganate; acetic anhydride;
In
acetone;
for 2h;
for 2h;
Alkaline conditions;
Reflux;
With
hydrogenchloride;
In
water;
|
61% |
|
sclareol;
With
potassium permanganate; acetic anhydride;
In
acetone;
at 0 - 20 ℃;
With
water;
for 2h;
Reflux;
|
61% |
|
sclareol;
With
potassium permanganate;
In
acetone;
at 20 ℃;
for 0.5h;
Cooling with ice;
for 2h;
Reflux;
Alkaline conditions;
|
61% |
|
sclareol;
With
potassium permanganate; acetic anhydride;
In
acetone;
at 0 - 20 ℃;
With
potassium hydroxide;
In
water;
for 2h;
Reflux;
|
61% |
|
With
potassium permanganate; potassium hydrogensulfate;
In
water; acetone;
at 20 - 30 ℃;
for 6h;
|
58% |
|
With
potassium permanganate; potassium hydrogensulfate;
In
acetone;
at 20 ℃;
for 5h;
|
58% |
|
With
ruthenium trichloride; sodium hydroxide; calcium hypochlorite; tetrabutyl-ammonium chloride;
In
tetrachloromethane; water; acetonitrile;
at 40 ℃;
pH > 12;
|
54% |
|
With
ruthenium trichloride; calcium hypochlorite;
In
tetrachloromethane; water; acetonitrile;
|
54% |
|
With
ruthenium trichloride; sodium hydroxide; calcium hypochlorite; tetrabutylammomium bromide; isopropyl alcohol;
In
tetrachloromethane; water; acetonitrile;
1.) 30 deg C, 20 min; 2.) 0 to 20 deg C, 1 h;
|
53% |
|
Multi-step reaction with 4 steps
1.1: peracetic acid; NaOAc / ethyl acetate / 192 h / 20 °C
2.1: 1 N aq. NaOH / 2-methyl-propan-2-ol / 20 °C
2.2: H2SO4 / ethyl acetate / 2 h / 0 - 20 °C
3.1: 97 percent / NaIO4 / tetrahydrofuran; H2O / 16 h / 20 °C
4.1: 95 percent / peracetic acid / 50 - 55 °C
With
peracetic acid; sodium hydroxide; sodium periodate; sodium acetate;
In
tetrahydrofuran; water; ethyl acetate; tert-butyl alcohol;
2.1: Payne rearrangement;
|
|
|
sclareol;
With
oxygen; ozone; sodium hydroxide;
In
water; acetic acid;
at 20 - 30 ℃;
for 5h;
With
sodium hydroxide;
In
tetrahydrofuran; water;
at 55 ℃;
With
hydrogenchloride;
In
tetrahydrofuran; water;
pH=1;
Time;
|
80 g |
-
-
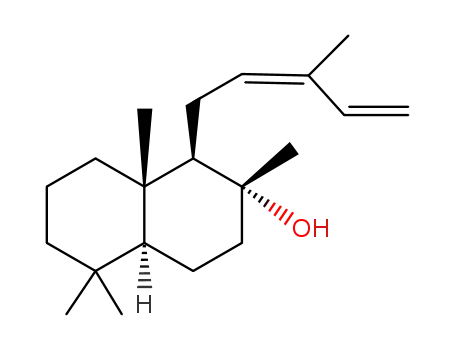
1616-86-0
cis-abienol

-
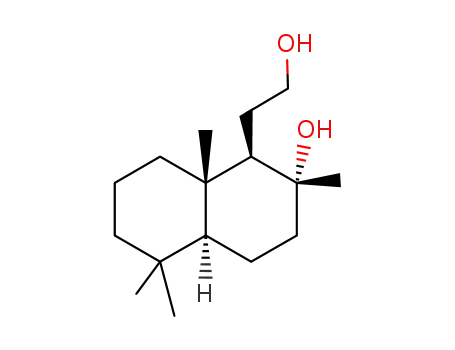
-
38419-75-9
(1R,2R,4aS,8aS)-1-(2-hydroxyethyl)-2,5,5,8a-tetramethyl-decahydronaphthalen-2-ol

-

-
564-20-5,1216-84-8,30450-17-0,58976-28-6,64090-92-2,67844-42-2,79768-41-5,79768-42-6,86688-27-9,86688-28-0,95406-08-9,98719-44-9,98719-46-1,131831-65-7,140851-80-5
(3aR)-(+)-sclareolide
| Conditions | Yield |
|---|---|
|
cis-abienol;
With
ozone;
In
ethanol;
at -20 - -15 ℃;
for 0.5h;
With
sodium tetrahydroborate;
In
ethanol;
at -20 - 5 ℃;
Temperature;
|
88% 11% |
|
Multi-step reaction with 2 steps
1.1: palladium(II) acetylacetonate; potassium hydroxide; water / toluene / 20 h / 0 - 20 °C
2.1: ozone / ethanol / 0.5 h / -20 - -15 °C
2.2: -20 - 5 °C
With
palladium(II) acetylacetonate; water; ozone; potassium hydroxide;
In
ethanol; toluene;
|
564-20-5 Upstream products
-
13456-36-5
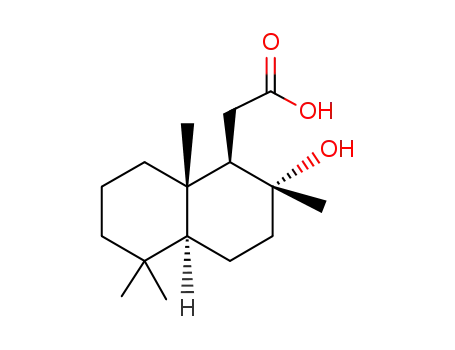
(1R,2R,4aS,8aS)-(2-hydroxy-2,5,5,8a-tetramethylperhydronaphthyl)acetic acid
-
473-03-0
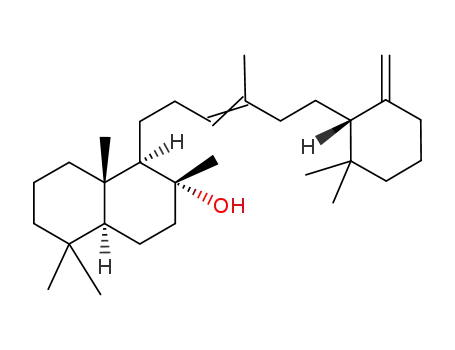
Ambrein
-
64681-71-6
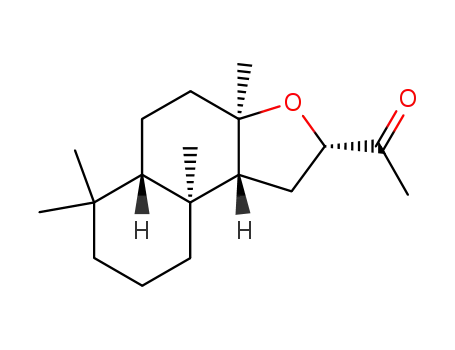
14,15-bisnor-8α,12S-epoxylabdan-13-one
-
1616-86-0

cis-abienol
564-20-5 Downstream products
-
38419-75-9

(1R,2R,4aS,8aS)-1-(2-hydroxyethyl)-2,5,5,8a-tetramethyl-decahydronaphthalen-2-ol
-
103476-92-2
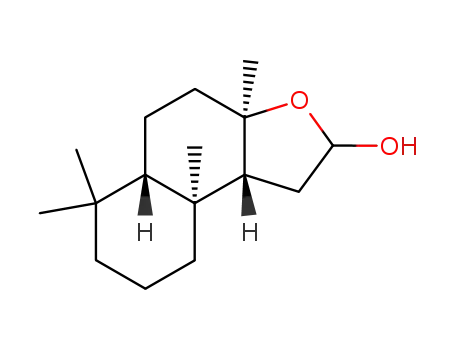
(3aR,5aS,9aS,9bR)-3a,6,6,9a-tetramethyldodecahydronaphtho[2,1-b]furan-2-ol
-
216011-55-1
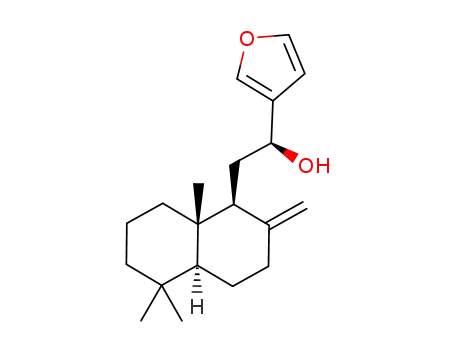
(5S,9S,10S)-15,16-epoxy-12-hydroxy-labda-8(17)-13(16),14-triene
-
61597-55-5
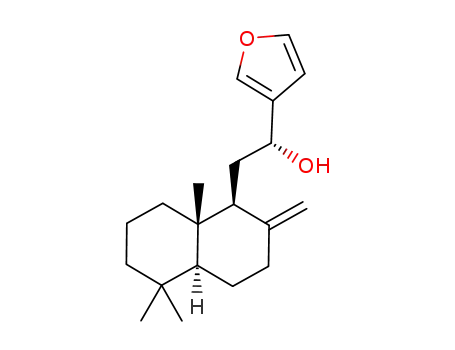
(5S,9S,10S)-15,16-epoxy-12-hydroxy-labda-8(17)-13(16),14-triene


