Cis-Jasmone
-
Product Name :
Cis-Jasmone
-
CAS No :
488-10-8
-
Project State :
Commercial
Application
General Description
Good factory supply good Cis-Jasmone 488-10-8
- Molecular Formula:C11H16O
- Molecular Weight:164.247
- Appearance/Colour:COA
- Vapor Pressure:0.0567mmHg at 25°C
- Melting Point:203 - 205oC
- Refractive Index:n20/D 1.498(lit.)
- Boiling Point:264.2 °C at 760 mmHg
- Flash Point:107.2 °C
- PSA:17.07000
- Density:0.937 g/cm3
- LogP:3.02210
Jasmone(Cas 488-10-8) Usage
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 39, p. 2317, 1974 DOI: 10.1021/jo00929a053Synthetic Communications, 5, p. 1, 1975 DOI: 10.1080/00397917508063507 |
|
Flammability and Explosibility |
Notclassified |
|
Synthesis |
A review and classification on the synthesis of jasmone is available. |
|
Definition |
A ketone found in jasmine oil and other flower oils. |
|
Taste threshold values |
Taste characteristics at 25 ppm: woody, bitter, tea, with a citrus and floral nuance. |
InChI:InChI=1/C10H14O/c1-3-4-5-9-8(2)6-7-10(9)11/h3-4H,5-7H2,1-2H3/b4-3-
488-10-8 Relevant articles
1,2-Bisanionic coupling approach to 2,3-disubstituted cyclopentenols and cyclopentenones
Luparia, Marco,Vadala, Alessandro,Zanoni, Giuseppe,Vidari, Giovanni
, p. 2147 - 2150 (2006)
We describe a new approach to 2,3-disubs...
Synthesis of Pyrethroids and Jasmonoids through Palladium-Catalyzed Cross-Coupling Reactions
Heguaburu, Viviana,Pandolfi, Enrique,Parpal, Florencia,Paullier, Ana Paula
, (2022/03/27)
The synthesis of jasmone and related jas...
Synthesis of Diketones, Ketoesters, and Tetraketones by Electrochemical Oxidative Decarboxylation of Malonic Acid Derivatives: Application to the Synthesis of cis-Jasmone
Ma, Xiaofeng,Dewez, Damien F.,Du, Le,Luo, Xiya,Markó, István E.,Lam, Kevin
, (2018/10/15)
Disubstituted malonic acid derivatives a...
Synthesis of Diketones, Ketoesters, and Tetraketones by Electrochemical Oxidative Decarboxylation of Malonic Acid Derivatives: Application to the Synthesis of cis-Jasmone
Ma, Xiaofeng,Du, Le,Luo, Xiya,Markó, István E.,Dewez, Damien F.,Lam, Kevin
, p. 12044 - 12055 (2019/03/01)
Disubstituted malonic acid derivatives a...
488-10-8 Process route
-
-
4868-21-7
undec-8c-ene-2,5-dione

-
-
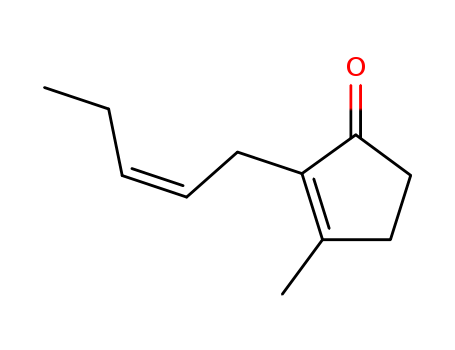
488-10-8
cis-jasmone

-
-
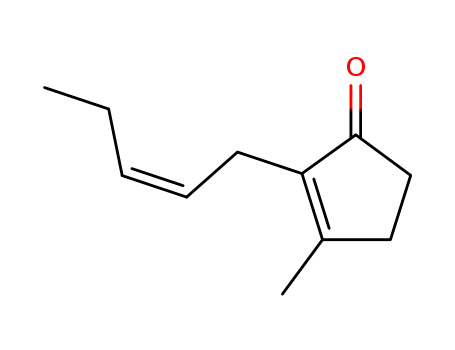
6261-18-3
trans-jasmone
| Conditions | Yield |
|---|---|
|
With
aluminum oxide;
In
benzene;
for 3h;
Heating;
|
60% |
-
-
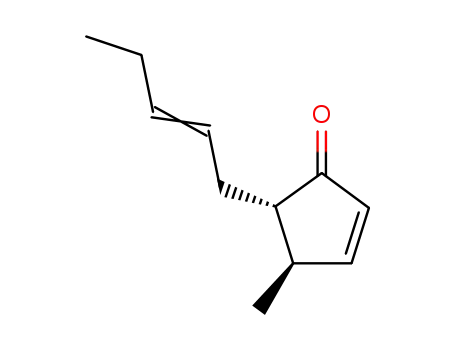
32556-66-4,32556-68-6,87392-23-2,131321-83-0,135029-97-9,135029-98-0
(4R,5S)-4-Methyl-5-((E)-pent-2-enyl)-cyclopent-2-enone

-

-
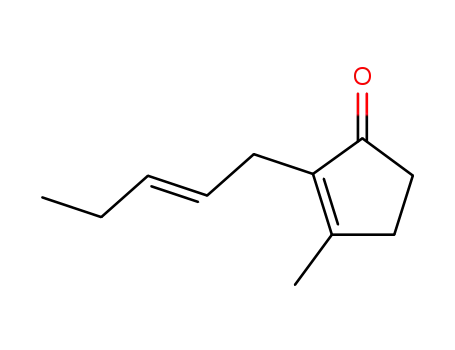
488-10-8
cis-jasmone

-

-
6261-18-3
trans-jasmone
| Conditions | Yield |
|---|---|
|
With
Amberlite IRA-400;
In
methanol;
for 24h;
Yield given. Yields of byproduct given. Title compound not separated from byproducts;
Ambient temperature;
|
488-10-8 Upstream products
-
53253-09-1
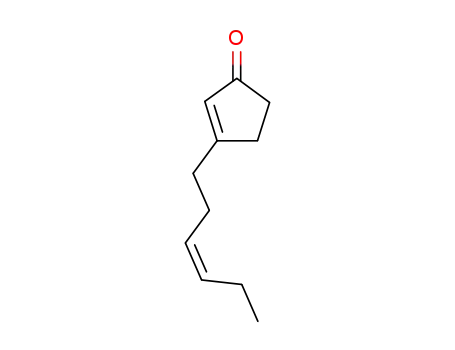
3-(cis-3-hexen-1-yl)-2-cyclopenten-1-one
-
80361-32-6
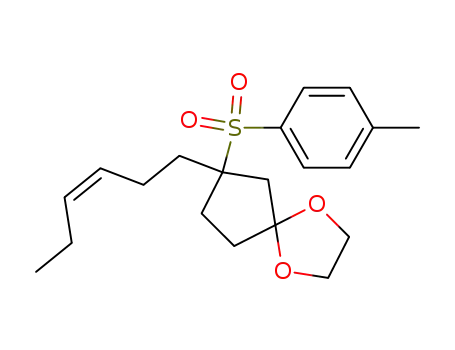
1,1-ethylenedioxy-3-(cis-hexen-1-yl)-3-(p-toluenesulfonyl)cyclopentane
-
81431-81-4
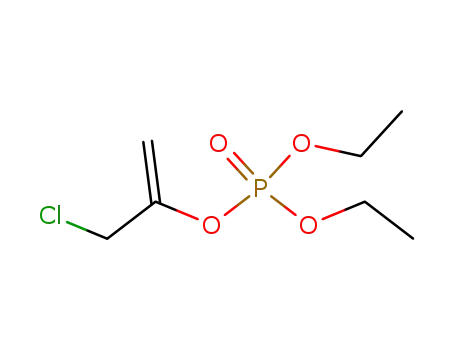
3-chloro-2-<(diethoxyphosphoryl)oxy>-1-propene
-
68776-91-0
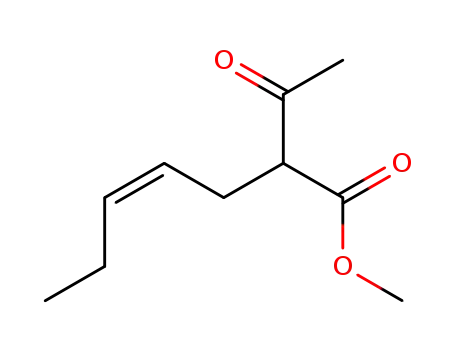
2-acetyl-(Z)-hept-4-enoic acid methyl ester
488-10-8 Downstream products
-
5009-20-1
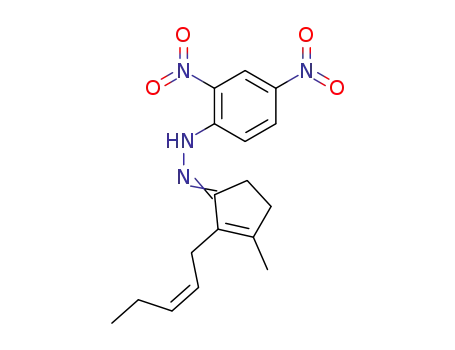
3-Methyl-2-(2-cis-pentenyl)-2-cyclopentenone (cis-Jasmone) 2,4-dinitrophenylhydrazone
-
1128-08-1
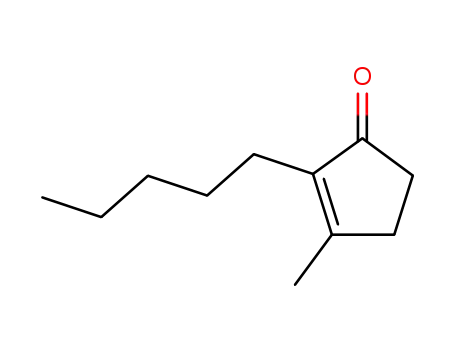
dihydro-cis-jasmone
-
23260-40-4
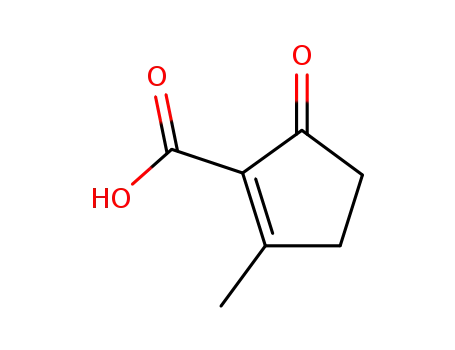
5-Methyl-5-cyclopenten-2-one-1-ylcarboxylic acid
-
123-76-2
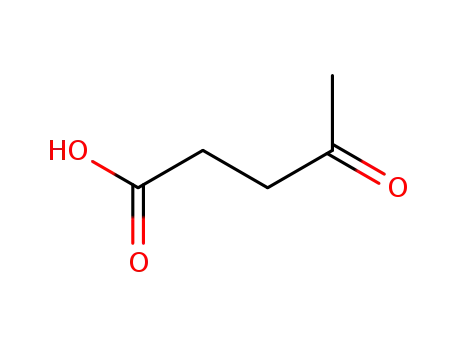
levulinic acid


