Ambrox Dl
-
Product Name :
Ambrox Dl
-
CAS No :
3738-00-9
-
Project State :
Commercial
Application
General Description
Cost-effective and customizable Ambrox Dl 3738-00-9 supplier
- Molecular Formula:C16H28 O
- Molecular Weight:236.398
- Appearance/Colour:White crystalline solid
- Melting Point:75-85ºC
- Boiling Point:273.9 ºC at 760 mmHg
- Flash Point:104.8 ºC
- PSA:9.23000
- Density:0.939 g/cm3
- LogP:4.40800
AMBROX DL(Cas 3738-00-9) Usage
|
Preparation |
Racemic sclareolide can be prepared by cyclization of homofarnesic acid in the presence of SnCl4 as a catalyst . Pure diastereomers are obtained by acid cyclization of (E)- and (Z)-4-methyl-6-(2,6,6-trimethylcyclohex-l(2)-enyl)-3- hexen-1-ol, prepared from 2-methyl-4-(2,6,6-trimethylcyclohex-l(2)-enyl)-2- butenal [394]. If the racemic sclareolide mixture is resolved into its enantiomers, the (–)-oxide may also be obtained by a totally synthetic route |
|
Synthesis Reference(s) |
Synthesis, p. 216, 1983 DOI: 10.1055/s-1983-30287 |
|
Trade name |
Compound starting from natural sclareol: Ambermore, Ambermore-DL, Ambermore-EX (Aromor), Ambrox? Super (Firmenich), Ambroxan? (Kao), Ambroxide (Symrise); compound starting from homofarnesic acid derivatives: Ambrox? DL (Firmenich); compound starting from 2-methyl- 4-(2,6,6-trimethylcyclohex-l(2)enyl)-2-butenal: Cetalox? (Firmenich), Cetalor (Aromor). |
InChI:InChI=1/C16H28O/c1-14(2)8-5-9-15(3)12(14)6-10-16(4)13(15)7-11-17-16/h12-13H,5-11H2,1-4H3
3738-00-9 Relevant articles
Synthesis and Applications of Cyclohexenyl Halides Obtained by a Cationic Carbocyclisation Reaction
Alonso, Pedro,Pardo, Pilar,Fontaneda, Raquel,Fa?anás, Francisco J.,Rodríguez, Félix
, p. 13158 - 13163 (2017/09/06)
The synthesis of cyclic alkenyl halides ...
Diastereoselective Synthesis of (±)-Ambrox by Titanium(III)-Catalyzed Radical Tandem Cyclization
Rosales, Antonio,Foley,Padial, Natalia M.,Muoz-Bascn, Juan,Sancho-Sanz, Iris,Roldan-Molina, Esther,Pozo-Morales, Laura,Iras-lvarez, Adriana,Rodrguez-Maecker, Roman,Rodrguez-Garca, Ignacio,Oltra, J. Enrique
, p. 369 - 374 (2016/02/09)
A synthesis of (±)-ambrox, a compound wi...
Scalable Synthesis of the Amber Odorant 9-epi-Ambrox through a Biomimetic Cationic Cyclization/Nucleophilic Bromination Reaction
Fontaneda, Raquel,Alonso, Pedro,Fa?anás, Francisco J.,Rodríguez, Félix
supporting information, p. 4626 - 4629 (2016/09/28)
A novel biomimetic nucleophilic bromocyc...
Synthesis of five-membered cyclic ethers by reaction of 1,4-diols with dimethyl carbonate
Aricó, Fabio,Tundo, Pietro,Maranzana, Andrea,Tonachini, Glauco
experimental part, p. 1578 - 1586 (2012/10/07)
The reaction of 1,4-diols with dimethyl ...
3738-00-9 Process route
-
-
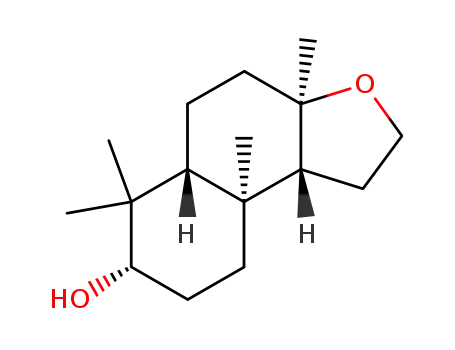
(d,l)-8α,12-epoxy-3β-hydroxy-13,14,15,16-tetranorlabdane

-
-
3738-00-9,6790-58-5,67844-43-3,68365-88-8,68365-89-9,100679-85-4,105561-31-7,108945-29-5,119818-38-1,122566-15-8,122566-16-9,122566-18-1,124578-52-5,131831-61-3,131831-62-4,131831-63-5,133907-53-6,138283-79-1,138283-80-4,145164-17-6
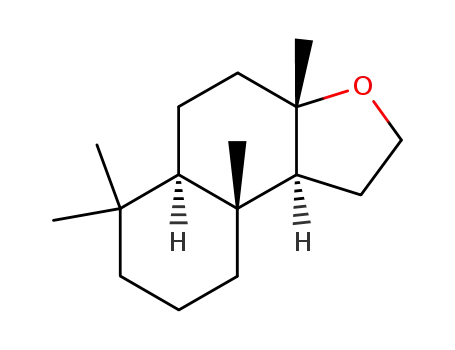
(±)-ambrox
| Conditions | Yield |
|---|---|
|
(d,l)-8α,12-epoxy-3β-hydroxy-13,14,15,16-tetranorlabdane;
With
dmap; pentafluorophenoxythiocarbonyl chloride;
In
dichloromethane;
at 0 - 20 ℃;
for 4h;
With
2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride;
In
benzene;
for 3h;
Reflux;
|
74% |
|
Multi-step reaction with 2 steps
1: 98 percent / trifluoromethanesulfonyl chloride, 4-DMAP / CH2Cl2 / 7 h / 0 °C
2: 100 percent / H2 / Pd/C / methanol / 760 Torr
With
dmap; trifluoromethane sulfonyl chloride; hydrogen;
palladium on activated charcoal;
In
methanol; dichloromethane;
|
-
-
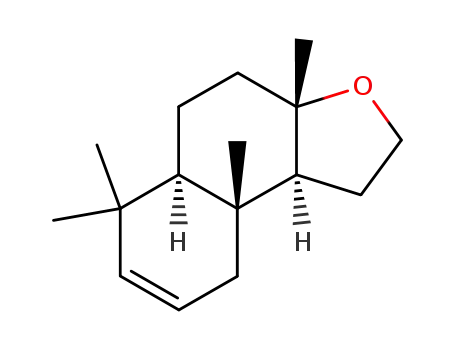
(d,l)-13,14,15,16-tetranor-8α,12-epoxy-2-labdene

-

-
3738-00-9,6790-58-5,67844-43-3,68365-88-8,68365-89-9,100679-85-4,105561-31-7,108945-29-5,119818-38-1,122566-15-8,122566-16-9,122566-18-1,124578-52-5,131831-61-3,131831-62-4,131831-63-5,133907-53-6,138283-79-1,138283-80-4,145164-17-6
(±)-ambrox
| Conditions | Yield |
|---|---|
|
With
hydrogen;
palladium on activated charcoal;
In
methanol;
under 760 Torr;
|
100% |
3738-00-9 Upstream products
-
138152-06-4
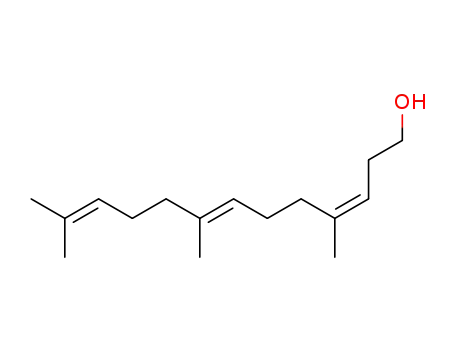
(3Z,7E)-4,8,12-Trimethyltrideca-3,7,11-trien-1-ol
-
459-89-2

(3E,7E)-homofarnesol
-
110202-08-9
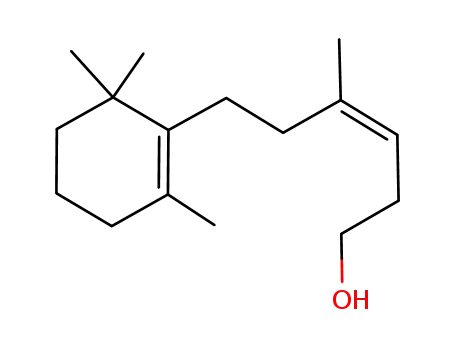
(Z)-4-Methyl-6-(2',6',6'-trimethyl-1'-cyclohexenyl)hex-3-en-1-ol
-
110202-07-8
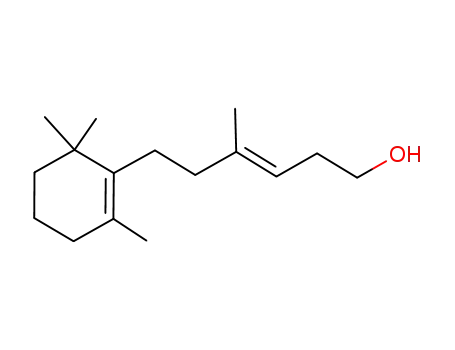
(E)-4-Methyl-6-(2',6',6'-trimethyl-1'-cyclohexenyl)hex-3-en-1-ol
3738-00-9 Downstream products
-
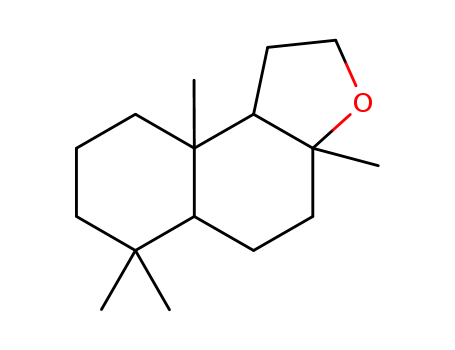
3738-00-9
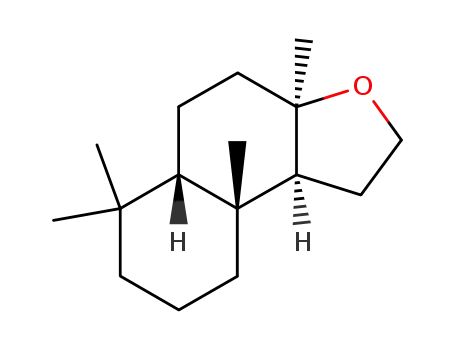
(3aRS,5aSR,9aRS,9bSR)-dodecahydro-3a,6,6,9a-tetramethylnaphtho[2,1-b]furan
-
3738-00-9
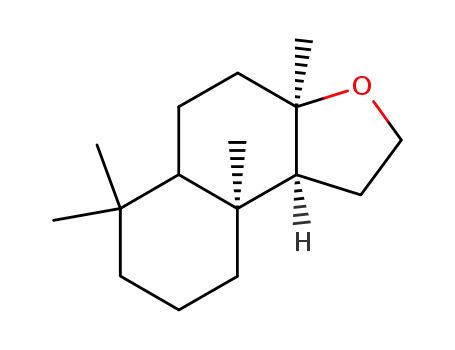
(-)-norlabdane
-
122566-19-2
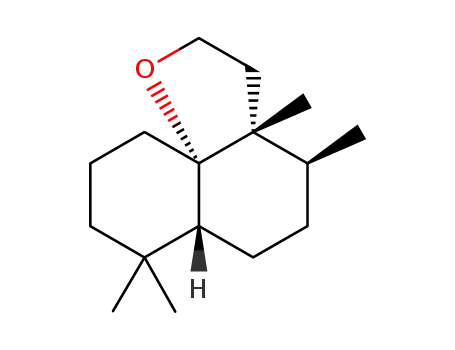
<3aR-(3aα,4α,6aα,10aS*)> dodecahydro-3a,4,7,7-tetramethyl-2H-naphtho<8a,1-b>furan


