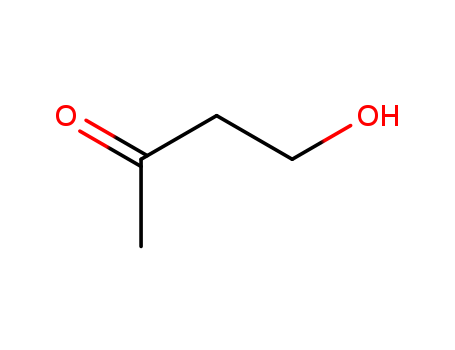
4-Hydroxy-2-butanone
-
Product Name :
4-Hydroxy-2-butanone
-
CAS No :
590-90-9
-
Project State :
Commercial
Application
General Description
Buy high quality and low price 4-Hydroxy-2-butanone 590-90-9 now
- Molecular Formula:C4H8O2
- Molecular Weight:88.1063
- Appearance/Colour:Colorless liquid
- Vapor Pressure:1.05mmHg at 25°C
- Melting Point:15°C (estimate)
- Refractive Index:1.430
- Boiling Point:156.3 °C at 760 mmHg
- PKA:14.43±0.10(Predicted)
- Flash Point:55.5 °C
- PSA:37.30000
- Density:0.987 g/cm3
- LogP:-0.04220
4-Hydroxy-2-butanone(Cas 590-90-9) Usage
|
Definition |
ChEBI: A beta-hydroxy ketone that is butan-2-one substituted by a hydroxy group at position 4. |
|
General Description |
4-Hydroxy-2-butanone is an important pharmaceutical intermediate. Gas phase reaction of the OH radicals with 4-hydroxy-2-butanone has been investigated by absolute rate method. |
InChI:InChI=1/C4H8O2/c1-4(6)2-3-5/h5H,2-3H2,1H3
590-90-9 Relevant articles
Chemoselective Oxidation of Secondary Hydroxy Groups by the Molybdenum Hexacarbonyl/Cetylpyridinium Chloride/t-Butyl Hydroperoxide System
Yamawaki, Kazumasa,Yoshida, Tsutomu,Suda, Takashi,Ishii, Yasutaka,Ogawa, Masaya
, p. 59 - 60 (1986)
The system molybdenum hexacarbonyl/cetyl...
Enzymatic syntheses of 13C-enriched geranylgeranyl diphosphate and casbene from 13C-labeled isopentenyl diphosphate
Huang, Qiulong,Huang, Kexue,Scott
, p. 2033 - 2036 (1998)
Geranylgeranyl diphosphate and casbene w...
HIGHLY SELECTIVE OXIDATION OF SECONDARY HYDROXYL FUNCTIONS USING THE VO(acac)2-t-BuOOH SYSTEM
Kaneda, Kiyotomi,Kawanishi, Yasuyuki,Jitsukawa, Koichiro,Teranishi, Shiichiro
, p. 5009 - 5010 (1983)
The VO(acac)2-t-BuOOH system shows high ...
Process for preparing 4-hydroxy-2-butanone
-
Paragraph 0024-0044, (2020/12/31)
The invention provides a process for pre...
Hydroxycarbonyl compound (by machine translation)
-
Paragraph 0070, (2020/01/31)
After the separated and purified efficie...
Preparation method of hydroxyl ketone compound
-
Paragraph 0024-0036; 0039-0048; 0055-0059, (2020/07/12)
The invention discloses a preparation me...
Reductive Electrochemical Activation of Molecular Oxygen Catalyzed by an Iron-Tungstate Oxide Capsule: Reactivity Studies Consistent with Compound i Type Oxidants
Bugnola, Marco,Shen, Kaiji,Haviv, Eynat,Neumann, Ronny
, p. 4227 - 4237 (2020/05/05)
The reductive activation of molecular ox...
590-90-9 Process route
-
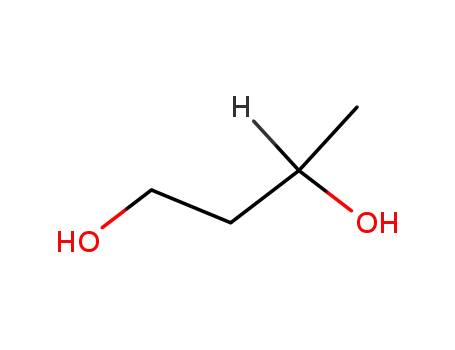
-
18826-95-4,107-88-0
1.3-butanediol

-
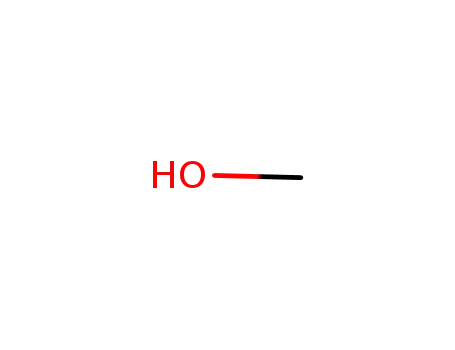
-
67-56-1
methanol

-
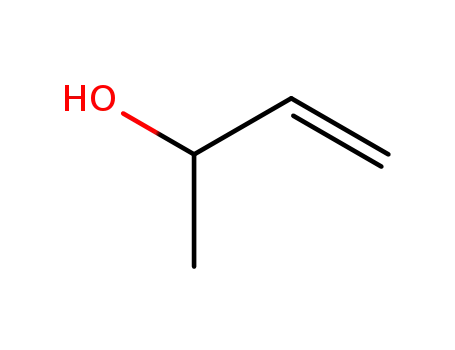
-
598-32-3
2-hydroxy-3-butene

-
-
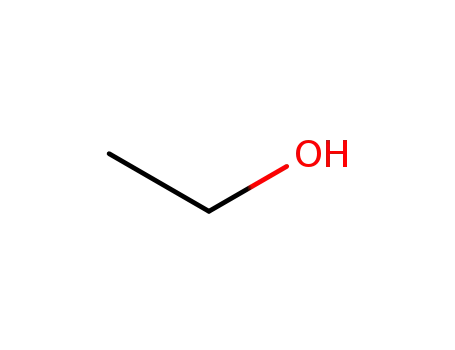
64-17-5
ethanol

-
-
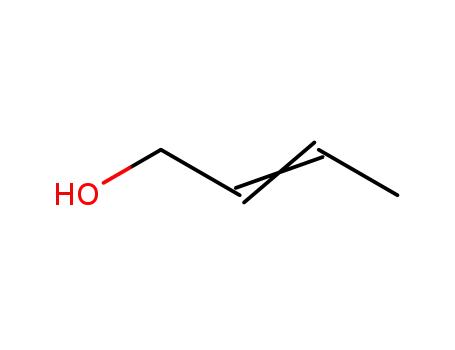
6117-91-5,542-72-3
(E/Z)-2-buten-1-ol

-
-
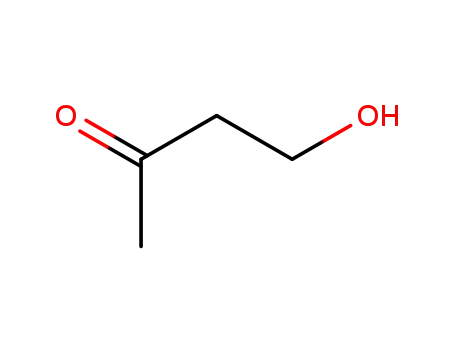
590-90-9
1-Hydroxy-3-butanone

-
-
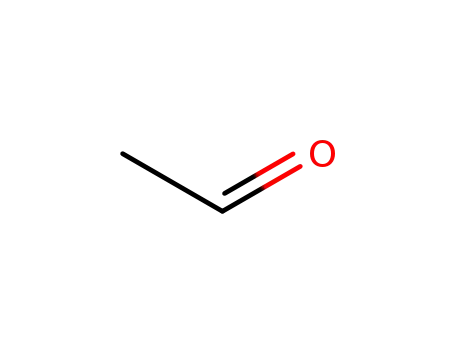
75-07-0,9002-91-9
acetaldehyde

-
-
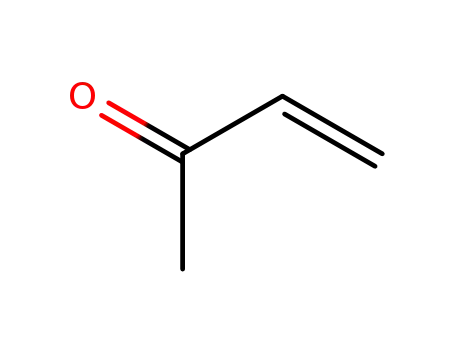
78-94-4,25038-87-3
methyl vinyl ketone

-
-
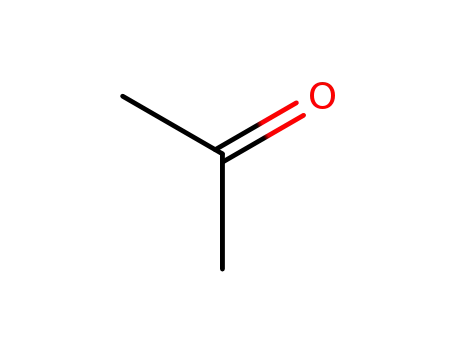
67-64-1
acetone

-
-
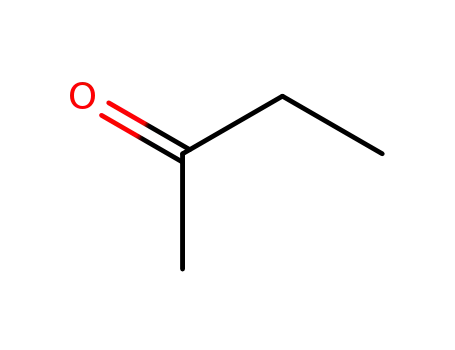
78-93-3
butanone

-
-
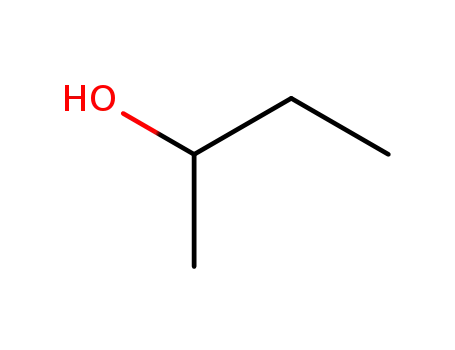
78-92-2,15892-23-6
iso-butanol

-
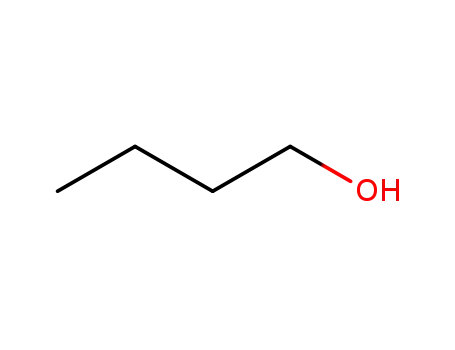
-
71-36-3
butan-1-ol
| Conditions | Yield |
|---|---|
|
In
neat (no solvent, gas phase);
under 759.826 Torr;
|
-

-
64-17-5
ethanol

-
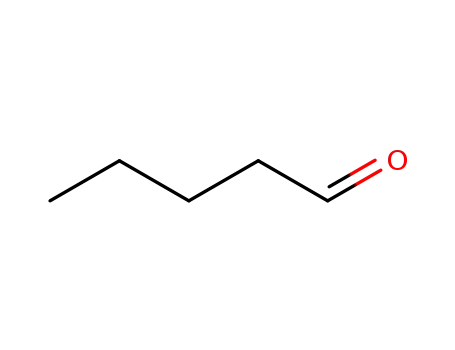
-
110-62-3
pentanal

-

-
590-90-9
1-Hydroxy-3-butanone

-

-
75-07-0,9002-91-9
acetaldehyde

-
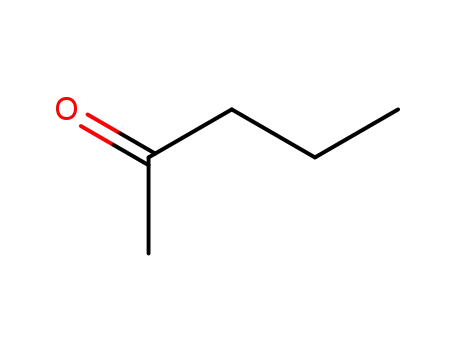
-
107-87-9
2-Pentanone

-
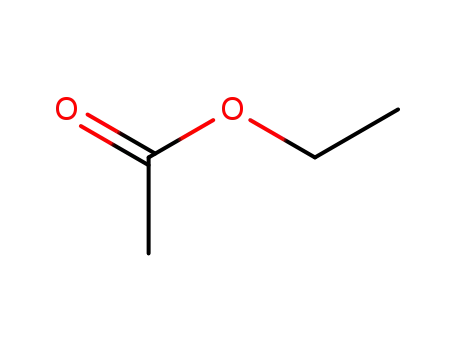
-
141-78-6
ethyl acetate

-
-
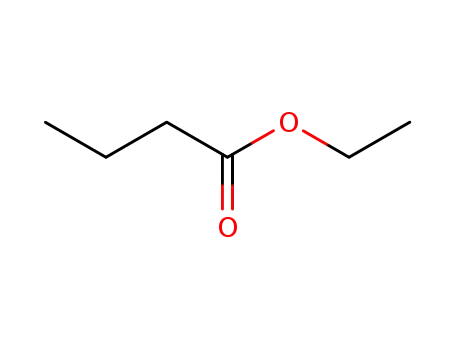
105-54-4
butanoic acid ethyl ester

-

-
67-64-1
acetone

-

-
78-93-3
butanone

-

-
71-36-3
butan-1-ol

-
-
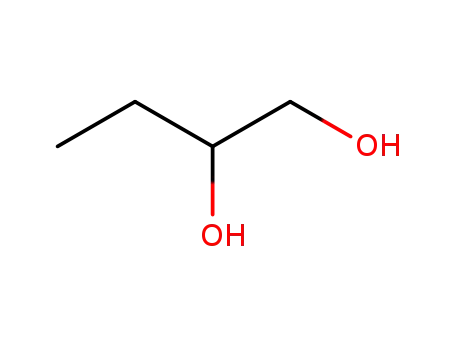
584-03-2
1,2-dihydroxybutane
| Conditions | Yield |
|---|---|
|
With
MgO/Cu;
at 300 ℃;
for 4h;
|
590-90-9 Upstream products
-
75-21-8
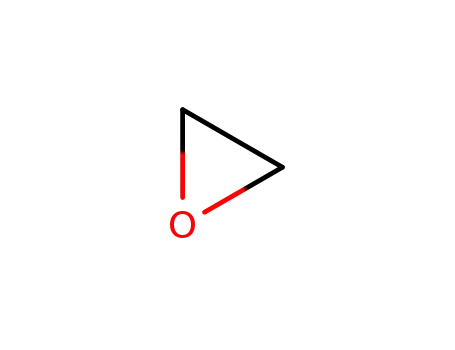
oxirane
-
75-07-0

acetaldehyde
-
689-97-4
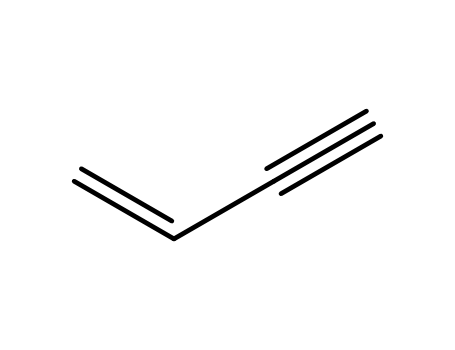
3-buten-1-yne
-
18826-95-4

1.3-butanediol
590-90-9 Downstream products
-
20705-59-3
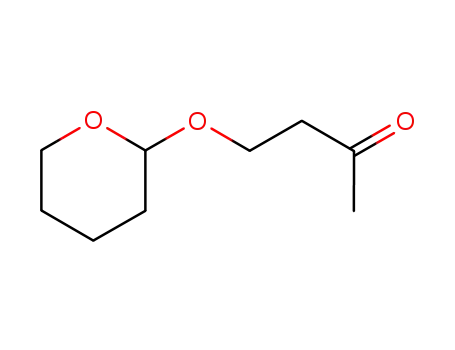
4-(tetrahydropyran-2-yloxy)butan-2-one
-
10150-87-5
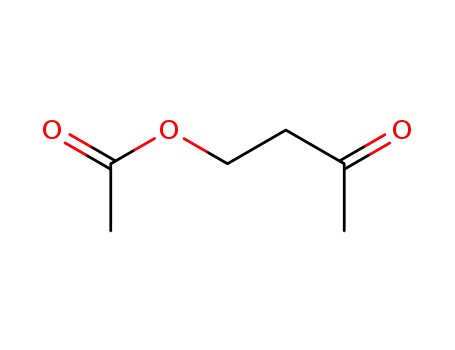
1-acetoxybutan-3-one
-
2568-33-4
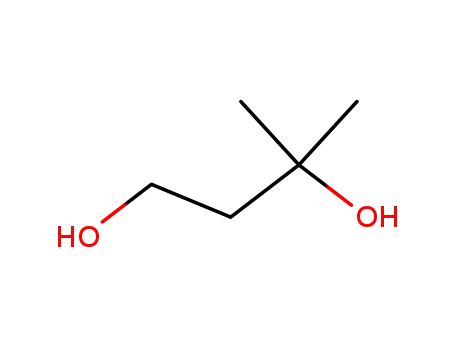
3-methyl-butane-1,3-diol
-
1192-42-3
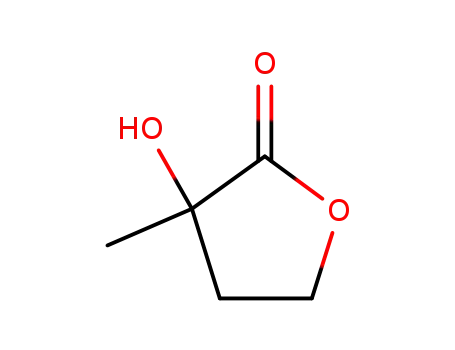
4,5-dihydro-3-hydroxy-3-methyl-2(3H)-furanone


