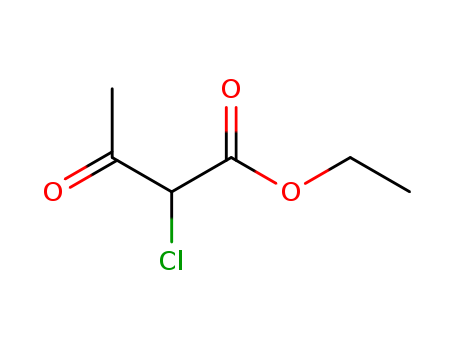
Ethyl 2-chloroacetoacetate
-
Product Name :
Ethyl 2-chloroacetoacetate
-
CAS No :
609-15-4
-
Project State :
Commercial
Application
General Description
Buy high quality and low price Ethyl 2-chloroacetoacetate 609-15-4 now
- Molecular Formula:C6H9ClO3
- Molecular Weight:164.589
- Appearance/Colour:clear bright yellow liquid
- Vapor Pressure:0.086mmHg at 25°C
- Melting Point:-80 °C
- Refractive Index:n20/D 1.441(lit.)
- Boiling Point:225.5 °C at 760 mmHg
- PKA:7.92±0.46(Predicted)
- Flash Point:50 °C
- PSA:43.37000
- Density:1.176 g/cm3
- LogP:0.74590
Ethyl 2-chloroacetoacetate(Cas 609-15-4) Usage
|
Biochem/physiol Actions |
Ethyl 2-chloroacetoacetate reacts with thiosemicarbazones to form heterocyclic substituted thiophene derivatives having non-steroidal anti-inflammatory activity. |
InChI:InChI=1/C6H9ClO3/c1-3-10-6(9)5(7)4(2)8/h5H,3H2,1-2H3/t5-/m1/s1
609-15-4 Relevant articles
ELECTROCHEMICAL CHLORINATION OF ETHYL ACETOACETATE AND THE FORMATION OF ETHYL 2,2,4-TRICHLOROACETOACETATE
Ilyushin, V. A.,Malaev, V. G.
, p. 756 - 757 (1988)
-
Synthesis, X-ray crystallographic analysis, DFT studies and biological evaluation of triazolopyrimidines and 2-anilinopyrimidines
Alsherbiny, Muhammad A.,Canfield, Peter,Fares, Mohamed,Gale, Philip A.,Guang Li, Chun,Jochmans, Dirk,Keller, Paul A.,Lewis, William,Neyts, Johan,Willis, Anthony C.
, (2021/12/21)
Inspired by the reported antiviral activ...
Development of isatin-thiazolo[3,2-a]benzimidazole hybrids as novel CDK2 inhibitors with potent in vitro apoptotic anti-proliferative activity: Synthesis, biological and molecular dynamics investigations
Eldehna, Wagdy M.,El Hassab, Mahmoud A.,Abo-Ashour, Mahmoud F.,Al-Warhi, Tarfah,Elaasser, Mahmoud M.,Safwat, Nesreen A.,Suliman, Howayda,Ahmed, Marwa F.,Al-Rashood, Sara T.,Abdel-Aziz, Hatem A.,El-Haggar, Radwan
supporting information, (2021/03/15)
In the current medical era, human health...
Design, synthesis, and insecticidal/acaricidal evaluation of novel pyrimidinamine derivatives containing phenyloxazole moiety
Zhang, Ning,Huang, Ming-Zhi,Liu, Ai-Ping,Liu, Min-Hua,Li, Li-Zhong,Zhou, Chun-Ge,Ren, Ye-Guo,Ou, Xiao-Ming,Long, Chu-Yun,Sun, Jiong,Dang, Ming-Ming,Lan, Zhi-Li
, p. 963 - 970 (2019/11/03)
A series of novel pyrimidinamine derivat...
Pyrimido-pyrrolopyridazine derivative as well as intermediate, preparation method, pharmaceutical composition and application
-
Paragraph 0086; 0094-0095, (2020/02/17)
The invention discloses a pyrimido-pyrro...
609-15-4 Process route
-
-
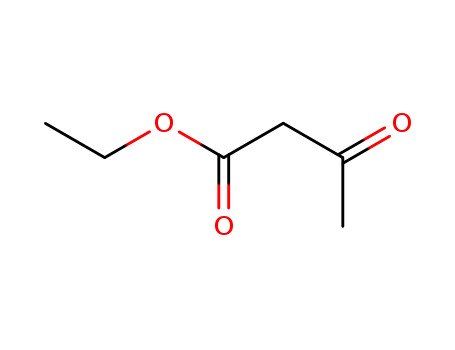
141-97-9
ethyl acetoacetate

-
-
88-00-6
ethyl 2,4-dichloro-3-oxobutanoate

-
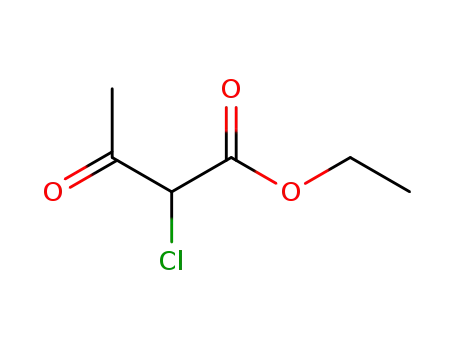
-
609-15-4
ethyl 2-chloro-3-oxo-butyrate
| Conditions | Yield |
|---|---|
|
With
chlorine;
at 35 ℃;
for 10h;
under 750.075 Torr;
Product distribution / selectivity;
|
-

-
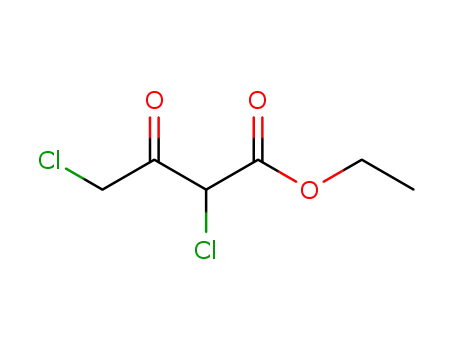
141-97-9
ethyl acetoacetate

-
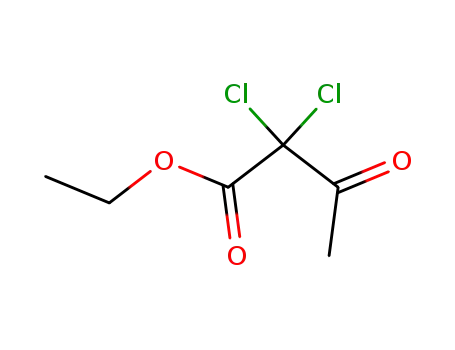
-
6134-66-3
ethyl 2,2-dichloroacetoacetate

-

-
609-15-4
ethyl 2-chloro-3-oxo-butyrate
| Conditions | Yield |
|---|---|
|
With
potassium peroxymonosulfate; ammonium chloride;
In
methanol;
at 20 ℃;
for 6h;
|
66 %Chromat. 12 %Chromat. |
|
With
chloro-trimethyl-silane; [bis(acetoxy)iodo]benzene;
In
acetonitrile;
at 20 ℃;
for 2h;
|
609-15-4 Upstream products
-
674-82-8
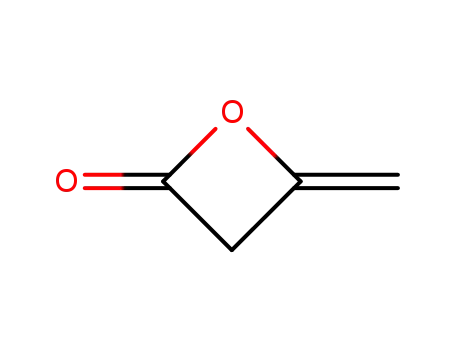
4-methyleneoxetan-2-one
-
64-17-5
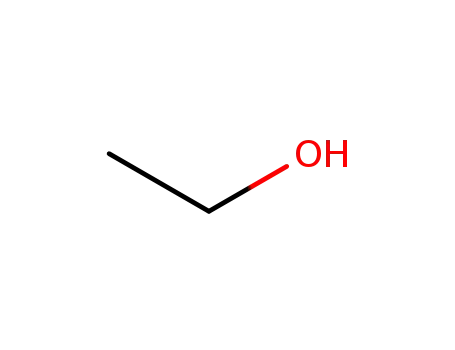
ethanol
-
67-66-3
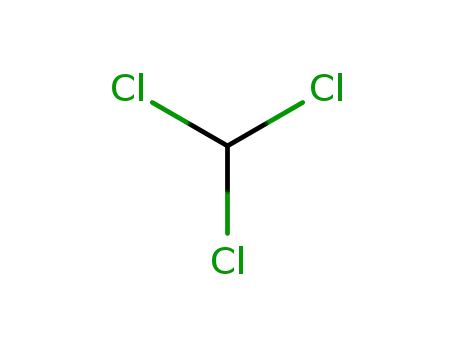
chloroform
-
112160-74-4
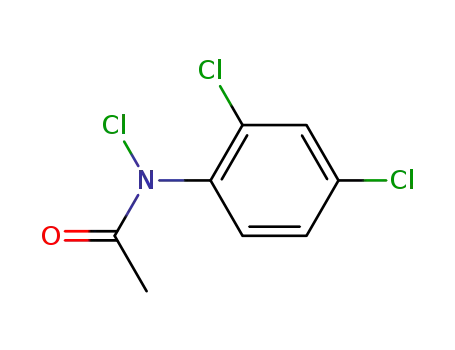
acetic acid-(2,4,N-trichloro-anilide)
609-15-4 Downstream products
-
41081-76-9
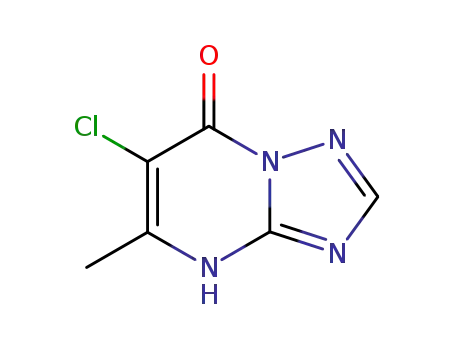
6-chloro-5-methyl-4H-[1,2,4]triazolo[1,5-a]pyrimidin-7-one
-
111562-39-1
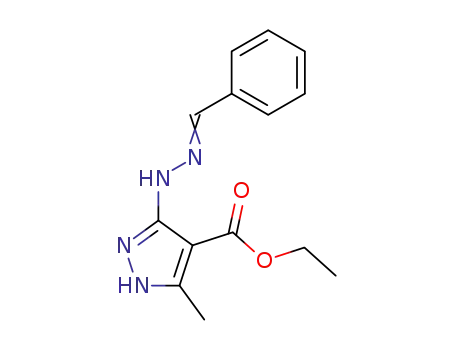
3-benzylidenehydrazino-5-methyl-1(2)H-pyrazole-4-carboxylic acid ethyl ester
-
897007-10-2
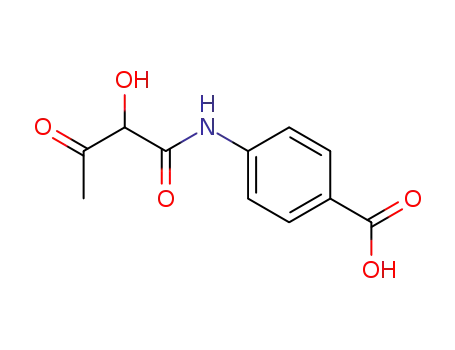
4-(2-hydroxy-acetoacetylamino)-benzoic acid
-
20582-55-2
ethyl 4-methylthiazole-5-carboxylate


